Pourquoi se joindre à
CellCarta?

Si vous êtes passionné par la science, en quête de nouveaux défis dans un milieu de travail dynamique et hautement collaboratif, joignez-vous à CellCarta et contribuez à façonner l’avenir de la médecine de précision.
CellCarta est un laboratoire de recherche contractuelle de premier plan dans le monde et son siège social est au Canada. Notre mission est de transformer la médecine de précision en offrant des solutions complètes pour l’analyse et la recherche de biomarqueurs aux plus importantes sociétés pharmaceutiques et biotechnologiques internationales. Notre objectif est de fournir des résultats scientifiques de haute qualité grâce aux partenariats proactifs que nous établissons avec nos clients.
Une équipe scientifique qui respecte les normes de qualité les plus rigoureuses
À la différence des organismes de recherche contractuelle classiques, nous avons une approche de scientifique à scientifique et notre culture se définit par la souplesse, l’accessibilité et l’excellence du service. Nous recherchons des passionnés de science animés par l’innovation et désireux de se joindre à une équipe de scientifiques diversifiée.
Chez CellCarta, nous nous sommes engagés à donner le meilleur à notre équipe. Joignez-vous à nous dans notre expansion à l’international et faites partie de l’avenir de la médecine de précision.
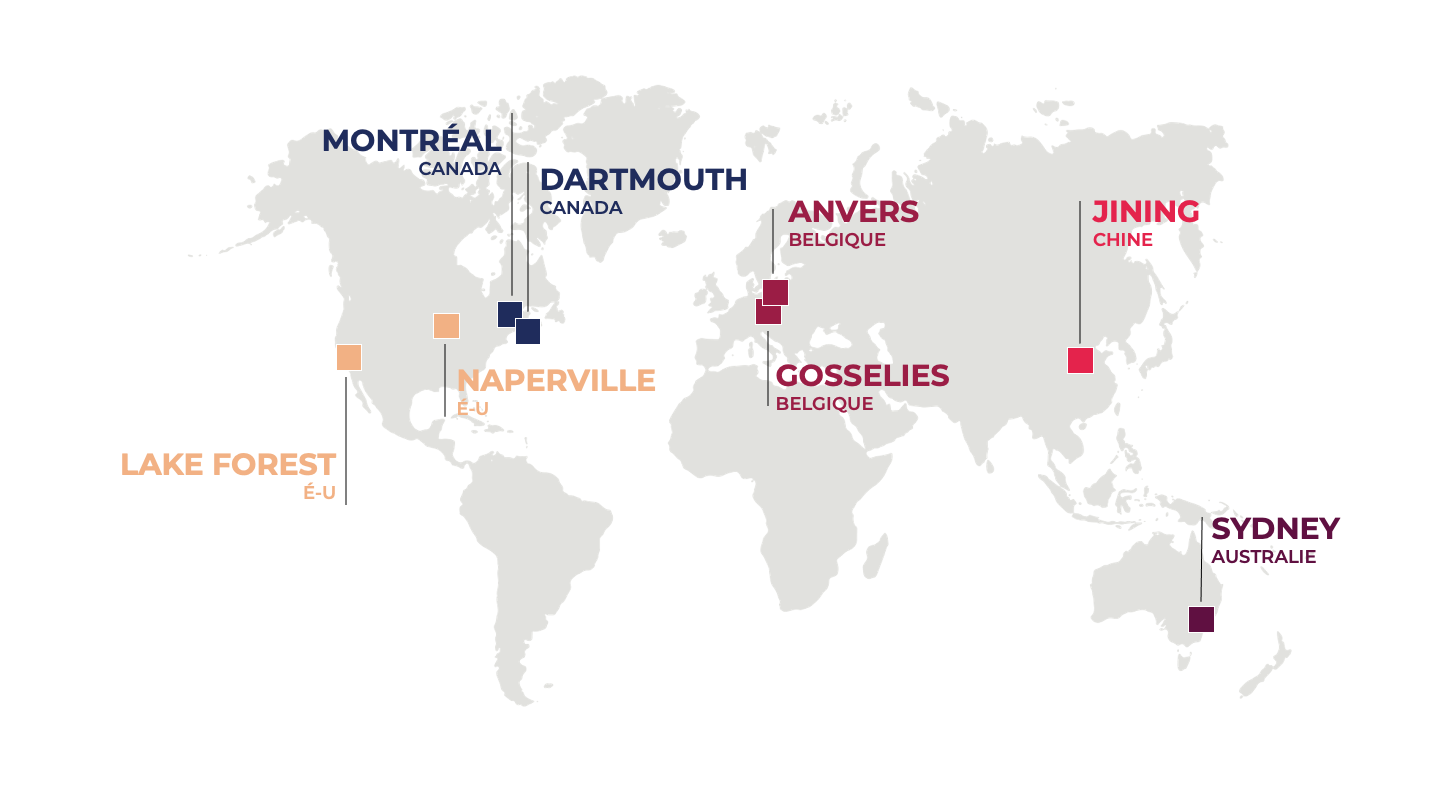
Nos Établissements :
ALLEZ PLUS LOIN
- Un environnement scientifique exceptionnel et stimulant au sein d’une équipe d’experts multidisciplinaire
- De multiples occasions de croissance et un plan de carrière
ALLEZ DE L’AVANT EN TOUTE CONFIANCE
- L’assurance que procure le fait de travailler pour un chef de file reconnu
- La réputation d’une équipe scientifique qui respecte les normes de qualité les plus rigoureuses
FAITES UNE DIFFÉRENCE
- Vous aurez la possibilité de contribuer de manière significative à la nouvelle génération de médicaments révolutionnaires de la médecine de précision
- Joignez-vous à une organisation de recherche contractuelle d’envergure mondiale où votre expertise compte et vos efforts ont un impact
Nos valeurs :
- Collaboration
- Excellence du service à la clientèle
- Respect
- Mériter la confiance de nos clients et assumer nos responsabilités
Nos Opportunités d’Emploi
View all jobs