Optimizing PK Assays for Accurate Biologics Profiles with CellCarta
Advancing Drug Analysis: PK Insights through Mass Spectrometry
Pharmacokinetic (PK) studies are an integral part of the research and development of novel therapeutic drugs.
These assays investigate the mechanisms of drug absorption, biodistribution, metabolism, and excretion.
The pharmacokinetics of a drug play an important role in understanding its effectiveness and safety, as well as identifying the appropriate dosage for preclinical and clinical studies.
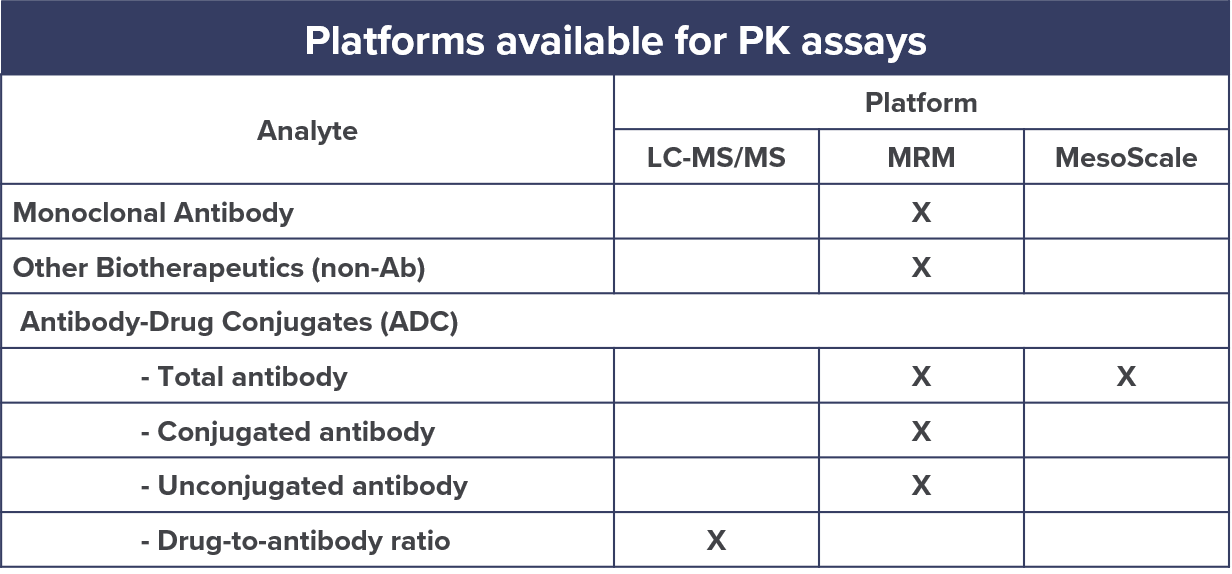
Different assays can be used to characterize PK properties of biologics both in the context of pre-clinical and clinical studies.
Mass spectrometry (MS)-based assays have the advantage of not requiring expensive and time-consuming antibody development which many other assays require.
They can also provide absolute quantification of biologics including monoclonal antibodies, antibody-drug conjugates, vaccines, blood products, and therapeutic proteins in various matrices.
At CellCarta, we perform the development, qualification, and validation of multiple reaction monitoring (MRM) based assays for PK analysis of biologics.
CellCarta for reliable, robust, and sensitive PK assays for your biologics
CellCarta has a proven track record for developing custom assays requiring complex sample preparation and MS analysis, while applying GCLP principles.
Our team of expert scientists will recommend the best platform for your PK analysis based on your specific needs, and our automated platforms will allow for high-throughput and reproducibility.
For additional convenience, our in-house bioinformatic and biostatistical team can provide detailed analysis of large data sets delivered as an easily interpretable report.
PK analysis: beyond the limitations of ELISA
ELISA is commonly used for PK analysis, but it has its limitations.
PK analysis performed with mass spectrometry has the advantage of specificity, fast method development and validation, and low sample amount requirements.
The technology also allows for the characterization of post-translational modifications (PTMs) and the easy transfer of an assay from animal model study to human sample analysis.