Careers
CellCarta: Precision Medicine Testing Services
If you are passionate about science, join CellCarta and help shape the future of precision medicine.

About us
As a global Contract Research Organization Laboratory (CRO) to the biopharmaceutical industry, CellCarta provides access to a broad offering of biomarker platforms and services. We partner with you to address the most complex scientific testing needs, delivering customized biomarker testing solutions to further the limitless potential of precision medicine.
CellCarta, which is under the private ownership of Arsenal Capital, is the union of scientific pioneers of the CRO industry supporting discovery, translational and clinical research. At the core of CellCarta is the fusion in 2019 of Caprion Biosciences and HistoGeneX which brought together a unique combination of biomarker expertise focused on immune monitoring, histopathology, proteomics and genomics, including the capabilities of previously acquired Primity Bio and Serametrix.
Leveraging its presence on four continents and through the addition of Clinical Logistics Inc. (since then renamed CellCarta Logistics), Mosaic Laboratories and Biogazelle (since then integrated within CellCarta NV), which further expanded its specimen logistics services and biomarker franchise, CellCarta is uniquely positioned to provide complex and customized clinical biomarker solutions at the global level.
At CellCarta, we are committed to staying at the forefront of scientific advancements, continually integrating the latest innovations into our service offerings.
Our adaptive approach ensures that we remain a trusted partner, ready to meet the evolving challenges of precision medicine with cutting-edge solutions.
At CellCarta, we understand that each individual is as unique and complex as a puzzle, an enigma waiting to be solved. Our scientists along with your team are on a journey of discovery, charting a course that leads to safer and more effective treatment solutions.
Our solutions are tailored to your needs and we always ensure that you benefit from our unparalleled scientific expertise at every stage of our collaboration with you.
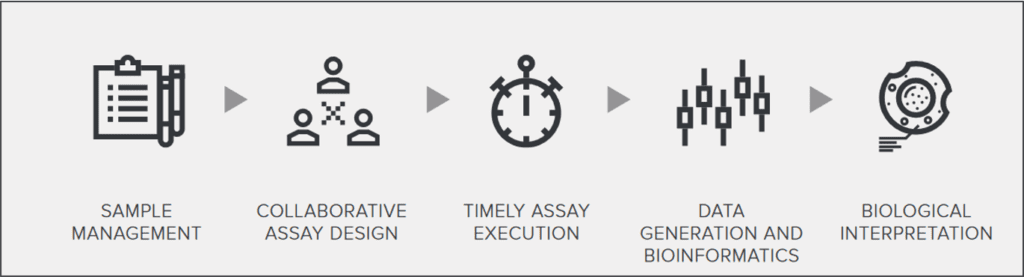
Working as an extension of your lab, our team of scientists has an unwavering commitment to proactively partnering with you in developing your biomarker strategies, using the most rigorous scientific controls.






With us as your partner, you too will go further. With access to our 4 integrated technological platforms and our end-to-end sample management services, we will be there, supporting you throughout the entire drug development cycle, from discovery to all phases of clinical development.
Explore our global & integrated laboratory testing services:
Immune Monitoring: Multiplex assays for monitoring of immune responses to support clinical trials.
Histopathology: Multiplexed tissue analysis with in-house team of pathologists for biomarkers, clinical testing, and companion diagnostics
Proteomics: Fully integrated mass spectrometry platforms designed for bioanalytical & clinical testing
Genomics: Proven and emerging molecular platforms for clinical sample analysis
At CellCarta, you’re not just joining a team—you’re becoming part of a global mission to transform precision medicine.
We cultivate a collaborative, innovative environment where career growth and the pursuit of scientific excellence go hand in hand, making a real impact on global therapeutic development.
Learn more about what CellCarta has to offer has an employer and discover opportunities in our laboratories across the world.
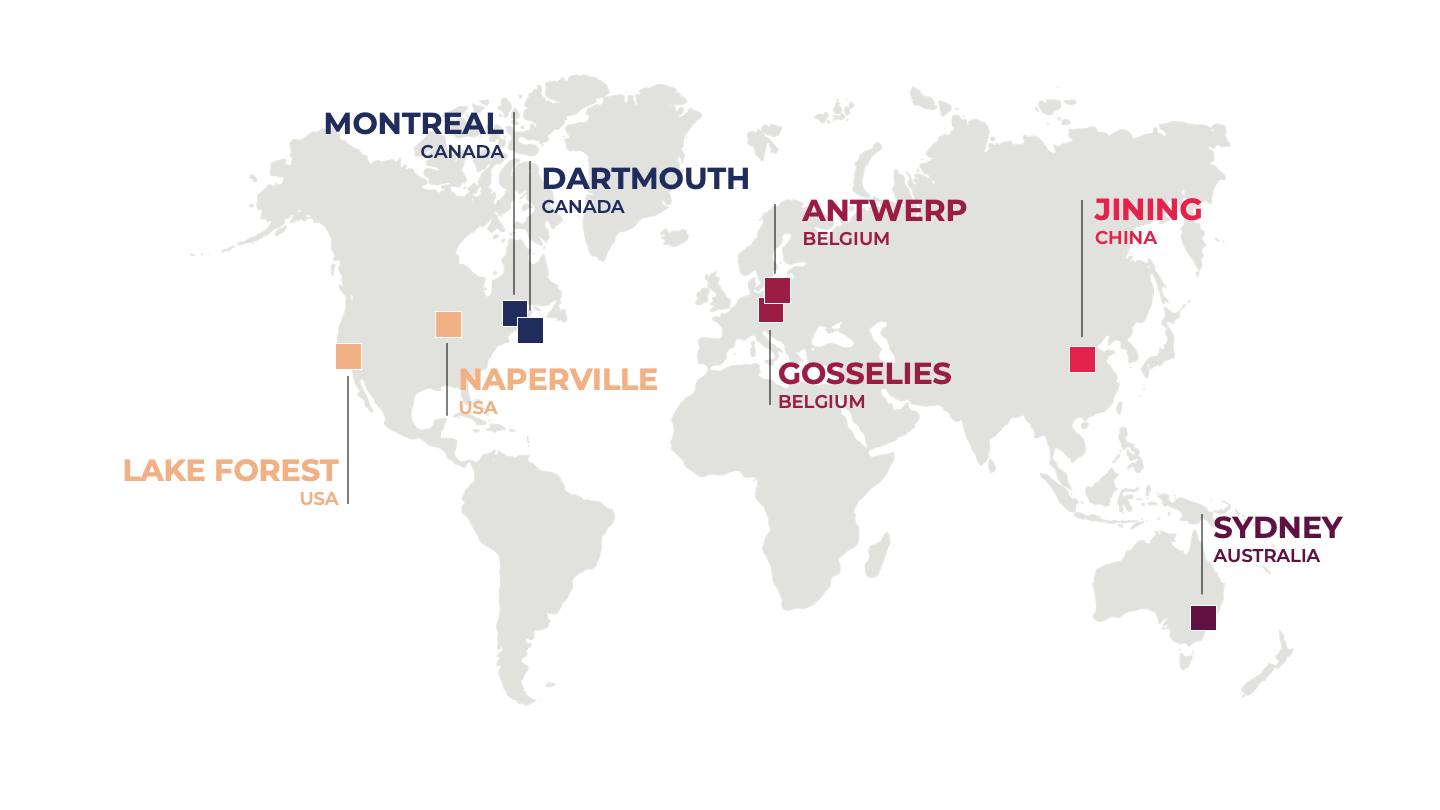
Headquartered in Montreal (Canada), the company operates globally with over 700 employees in its nine facilities located in Canada, USA, Belgium, Australia, and China.
To learn more about our China offering, click here.
Our global reach is strengthened by deep local expertise, allowing us to navigate region-specific regulatory environments.
This ensures that our global projects are tailored to meet the unique standards and needs of each region we serve.
Chief Executive Officer

Dusty is an accomplished executive with 20 years of experience within the life sciences industry. Prior to joining CellCarta as Chief Executive Officer, Dusty served as an Operating Partner for Arsenal Capital Partners healthcare team. Further, Dusty has led several business transformations and related strategic acquisitions as CEO of Stirling Ultracold, President of Brooks Automation’s life sciences division (now Azenta) and Sr. Vice President of PerkinElmer’s Environmental Health division.
Dusty earned his bachelor’s degree, and graduated cum laude, from the University of Maryland, and he received his Master of Science degree from the University of Vermont.
Chief Financial Officer

Tony is a seasoned financial executive with a proven background leading teams in a variety of business environments including strategic growth and operating model transformations, business acquisitions, and turnarounds. He joined Caprion in October 2020 and oversees the finance, accounting, tax, treasury, and legal functions of the global corporation.
Prior to joining Caprion, Tony was Senior Vice President and CFO at Albany Molecular Research, Inc. – a GTCR/Carlyle Group portfolio CDMO. In this role, he had leadership responsibilities for all aspects of finance, accounting, tax, treasury, pricing, global information systems, lender relations, acquisitions/divestitures, and corporate strategy. He also shared responsibility for several enterprise-wide projects. Prior to this, Tony served as CFO for Bruker Corporation, a publicly traded global scientific instrumentation provider. He also held other finance executive roles with Merck KGaA, Millipore, and Gerber Scientific. In addition to his corporate finance roles, he has also served as an auditor at Price Waterhouse.
Tony holds a BS in accounting from Central Connecticut State University, an MBA from the University of Connecticut, and is a CPA.
Chief Commercial Officer

Chief Scientific Business Officer

Mr. Christopher Ung has served in the companion diagnostics field since its inception and is one of the original pioneers of the personalized medicine field. He led the global commercialization efforts of the HercepTest™ and pharmDx EGFR companion diagnostic assays and continues to lead and implement global biomarker strategies for drug development projects. He has established several global companion diagnostic partnerships that have played a pivotal role in targeted therapy development and has set up CAP, CLIA laboratories in the US, Scotland, China, and Singapore.
Mr. Ung leads the development and execution of HistoGeneX’s strategic and business initiatives, leveraging the company’s solid tumor and anatomic pathology services. Prior to joining HistoGeneX, he was Vice President of Strategic Business & Operations at Quintiles, Chief Operating Officer for Targeted Molecular Diagnostics and Chairman of the Pathology Business Group at DAKO.
Chief Information Officer

Louis is a seasoned technology leader with over 25 years of experience in enterprise architecture, application development, and life sciences. Prior to joining CellCarta, Louis held various leadership roles at Labcorp and Covance, where he led multiple software engineering teams and managed global technology initiatives. Louis oversees CellCarta’s global technology functions, leveraging his deep experience in laboratory processes, IT service management, and solution architecture to support business operations.
Louis earned his BS in Chemistry from Canisius University and a PhD in Chemistry from the University of Illinois.
Senior Vice President, Immunology & Proteomics Business Units

Todd joined Caprion in September 2020 as the leader of our quality, regulatory and government affairs team, overseeing the overall regulatory requirements on ISO, CAP /CLIA, GLP and GCLP, and assisting clients in defining their global regulatory pathways for their development and clinical programs.
Todd brings more than 26 years of combined experience in the biopharmaceutical, diagnostics, and food/nutrition businesses at Abbott. Todd has held a variety of technical and leadership roles in R&D, Operations, QA, and Regulatory Affairs. He has served on a number of boards and academic advisory positions including Regulatory Affairs Professional Society (RAPS), Lambs Farm, the International Life Sciences Institute, the Dean’s Advisory Board for the School of Pharmacy at Purdue University and as a Ford Scholar at Northwestern’s Kellogg School of Management.
Todd earned his bachelor’s degree in Pharmacy from Purdue University, a master’s degree in Engineering and Management Sciences from Northwestern University and a PhD in Pharmacy from the University of Illinois.
Vice President, General Counsel and Corporate Secretary

Nancy El Sayegh joined CellCarta as Vice President, General Counsel and Corporate Secretary in September 2019. Nancy helps CellCarta navigate business and legal challenges while supporting its expansion as a global provider and industry leader specialized in precision medicine laboratory services. She also provides strategic guidance in areas such as cybersecurity, privacy, labor and employment and intellectual property, among others.
Beyond the legal sphere, Nancy oversees ESG and global facilities management, with her efforts dedicated towards optimizing physical infrastructure to enhance operational efficiency, sustainability, and employee well-being across the organization.
Senior Vice President, Data Science and Management

Ted received his PhD in Macromolecular, Cellular Structure and Chemistry from The Scripps Research Institute in La Jolla, California. He joined CellCarta in 2023 to lead the global data science and management function. Ted brings 20 years of bench R&D, bioinformatics, computational biology and data management experience to the role. He is focused on building a world-class data team in order to efficiently and effectively deliver CellCarta’s substantial and continually growing portfolio of advanced biomarker measurements, and leading the development of advanced data investigational capabilities to provide sponsors new tools for data comprehension and insight generation.
Prior to CellCarta, Ted was the VP of Biomarker Data Management and Systems Integration at QuartzBio, and has held prior leadership roles with PatientsLikeMe, Wuxi Nextcode, Courtagen Life Sciences, Life Technologies, and US Genomics. Ted graduated with a dual Bachelors in Chemistry and Psychology from Colgate University.
Chief Scientific Officer & Medical Director

Dr. Mark Kockx founded HistoGenex Laboratories in 2001. At the University of Antwerp, Belgium, Dr. Kockx pioneered methodologies to elucidate apoptotic and cytostatic response of cancer patients after treatments with targeted therapies. His subsequent collaborations with global pharmaceutical companies led to the founding of Histogenex Laboratories.
Dr. Kockx is an internationally recognized pathologist-scientist who has operated on the forefront of personalized medicine developments. He continues to lead initiatives to standardize biomarker analysis practices in addition to adapting novel technologies. Dr. Kockx has been extensively involved with the clinical trials that led to the approval of well-known targeted therapies such as EGFR, BRAF and MEK inhibitors. His current passions are two fold. He is introducing practices and workflows for next generation IHC, combining innovations of multiplex staining, quantification and image capture. He has also developed an Immunomics program, a state of the art biomarker program that supports the evolving and numerous needs of immunotherapy development.
Senior Vice President, Genomics

As the Founder and CEO of Sampled, a spin out of Rutgers University serving the Biopharma with early stage, discovery and translation genomics. Robin brings valuable expertise in genomics, automation-driven solutions, and integrated service models for diagnostics and therapeutic research.
Prior to joining CellCarta, Robin held executive positions at Azenta Life Sciences (formerly Brooks Automation), where he led automation, consumables, and instrument business units, driving substantial revenue growth and transforming sample management technologies. Azenta acquired FluidX where Robin was a founder and owner, developing novel consumables and Instruments for Sample Management and Hight Throughput Screening.
He currently serves in the ISBER (International Society for Biological and Environmental Repositories) Outstanding New Product Award Task Force. His contributions to automation, sample storage innovation, and genomics technology development are reflected in multiple patents and industry recognitions.
Robin studied Applied Biology with Biochemistry/Microbiology at Anglia Ruskin University and obtained an Executive Certificate in Management and Leadership from the MIT Sloan School of Management.
Chairman of the BoardOperating Partner, Arsenal Capital Partners
Chief Executive Officer of CellCarta
Vice-ChairmanCo-founder of CellCarta Biosciences
Operating Partner, Arsenal Capital Partners
Senior Partner, Arsenal Capital Partners
Investment Partner, Arsenal Capital Partners
Principal, Arsenal Capital Partners
Advisory Board Chairman & Senior Advisor, Arsenal Capital PartnersNobel Laureate
Senior Advisor & Advisory Board Member, Arsenal Capital Partners
Senior Advisor & Advisory Board Member, Arsenal Capital Partners
Co-Founder and Board Member
Co-Founder and Board MemberChief Scientific Officer & Medical Director, CellCarta
Chairman of Arsenal Capital Partners Healthcare Advisory Board
Nobel Laureate

Dr. Rothman is the Sterling Professor of Cell Biology and Chair of the Department of Cell Biology at Yale University School of Medicine. His research discoveries have been recognized by the Nobel Prize in Physiology or Medicine in 2013, the Albert Lasker Award and the Kavli Prize for Neuroscience. Dr. Rothman has been elected to membership in the National Academy of Sciences and its Institute of Medicine. Previously, he served as Chief Scientist of General Electric Healthcare and has served on the advisory boards of Amersham PLC, Johnson & Johnson Scientific, and Merck Scientific. Dr. Rothman graduated with a B.A. from Yale College, attended Harvard Medical School and received a Ph.D. from Harvard University. He has previously been a professor at Stanford, Princeton, and Columbia Universities and at Memorial Sloan- Kettering Cancer Center, where he served as Vice Chairman of the Sloan-Kettering Institute.
23rd Commissioner of the FDA

Dr. Gottlieb is a physician and served as the 23rd Commissioner of the U.S. Food and Drug Administration. Dr. Gottlieb’s work focuses on advancing public health through developing and implementing innovative approaches to improve medical outcomes, reshape healthcare delivery, and expand consumer choice and safety. He is currently a partner at the venture capital firm New Enterprise Associates; a resident fellow at the American Enterprise Institute; a contributor to CNBC; and a board member to Pfizer, Inc. and Illumina, Inc.
Under his leadership, the FDA advanced new frameworks for the safe and effective oversight of gene therapies, cell-based regenerative medicines, targeted drugs, digital health devices, and vaccine manufacturing. The agency implemented new reforms to standardize drug reviews and make historic improvements of post-market data collection and the use of real-world evidence. He promoted policies to reduce death and disease from tobacco, improve food innovation and safety, and aggressively confront addiction crises. The agency’s historic and prolific advances in new policy distinguished his tenure as the FDA’s commissioner, in addition to a record-setting number of approvals of novel drugs, medical devices, and generic medicines.
Previously, Dr. Gottlieb served as the FDA’s Deputy Commissioner for Medical and Scientific Affairs and before that, as a Senior Adviser to the Administrator of the Centers for Medicare and Medicaid Services, where he helped advance policies to improve healthcare quality and promote the effective use of new medical technologies. Dr. Gottlieb was a practicing hospitalist and a Clinical Assistant Professor at the New York University School of Medicine. He is an elected member of the National Academy of Medicine.
Department of Microbiology and Immunology, Stanford University

Dr. Nolan is the Rachford and Carlota A. Harris Professor in the Department of Microbiology and Immunology at Stanford University School of Medicine. He trained with Leonard Herzenberg (for his PhD) and Nobelist Dr. David Baltimore (for postdoctoral work on the first cloning/characterization of NF-κB p65/RelA and the development of 293T rapid retroviral production systems). He has published over 200 research papers, holds 17 US patents and has been honored as one of the top 25 inventors at Stanford University. He has trained more than 30 graduate students and 40 postdoctoral or clinical fellows. Dr. Nolan’s areas of research include hematopoiesis, cancer and leukemia, autoimmunity and inflammation, and computational approaches for network and systems immunology. His most recent efforts are focused on a single cell analysis advance using a mass spectrometry-flow cytometry hybrid device, called CyTOF. The approach uses an advanced ion plasma source to determine the levels of tagged reagents bound to cells—enabling a vast increase in the number of parameters that can be measured per cell. Another recent innovation is termed molecular ion beam imaging (MIBI), a system that also uses mass tags that will enable sub-light imaging (5 nm resolution) of tissue sections with 50 or more parameters per image. His laboratory has already begun a large-scale mapping of the hematopoietic hierarchy in healthy human bone marrow at an unprecedented level of detail. A large focus of his lab is the development and utilization of machine learning algorithms to interpret the large high-dimensional datasets being produced by CyTOF and MIBI. Dr. Nolan’s efforts are to enable a deeper understanding not only of normal immune function, trauma, and other inflammatory events but also detailed substructures of leukemias and solid cancers—which will enable wholly new understandings, permitting better management of disease and clinical outcomes.
Department of Pathology, Case Western Reserve University

Dr. Sekaly is the Richard J. Fasenmyer Professor of Immunopathogenesis at the Case Western Reserve University School of Medicine, Director of the Center for Systems Immunology, and member of the Hematopoietic and Immune Cancer Biology Program at the Case Western Comprehensive Cancer Center. Dr. Sekaly’s lab has been focused for the past 20 years on developing a better understanding of the human immune response to vaccines and to chronic viral infections, with a specific focus on cancer and HIV infection. Over the past 7 years, Dr. Sekaly and his team have implemented the use of system biology approaches to monitor the diversity of memory T cells and to identify the mechanisms underlying it. His group has also pioneered the application of systems biology approaches to the understanding of mechanisms of action of the licensed vaccines and adjuvants, and has shown for the first time the diversity of the mechanism that lead to the establishment and persistence of different memory T cell subsets. Dr. Sekaly’s work has led to more than 342 peer-reviewed articles in scientific journals and more than 23 patents. He has been a principal investigator on numerous grants from the National Institutes of Health and foundations, including the Bill and Melinda Gates Foundation and the American Foundation for AIDS Research.
Senior Vice President for Experimental Therapeutics and Director, Sarcoma Center, Dana-Farber Cancer Institute; Professor and Co-Director, Ludwig Center at Harvard

George Demetri, MD, FASCO has dedicated his career to translational research aimed at understanding and treating precisely-defined subsets of cancers. He was a pioneer in the development of imatinib (Gleevec®), the first cancer therapy targeting gastrointestinal stromal tumor (GIST) as a molecularly defined subset of sarcoma. Subsequently, his work has contributed to the U.S. FDA and worldwide regulatory approvals of several other “smart drugs” for cancer, including sunitinib (Sutent®) and regorafenib (Stivarga®) for GIST, tazemetostat (Tazverik®) for epithelioid sarcomas, as well as pazopanib (Votrient®) and trabectedin (Yondelis®) for other sarcomas. In a related contribution, Dr. Demetri served on the Scientific Advisory Board for Plexxikon to help develop the first mutant BRAF inhibitor, vemurafenib (Zelboraf®), a mutation-targeted therapy for a subset of melanomas. Most recently, he helped starting Blueprint Medicines in Cambridge, MA, which recently announced the U.S. FDA approval of avapritinib (Ayvakit®) for a mutationally-defined subset of GIST.
He received his undergraduate degree in Biochemistry from Harvard College, then was a research fellow at the Universite of Besancon, France before receiving his medical degree from Stanford University School of Medicine. Subsequently, he completed his residency and became Chief Residency in Internal Medicine at the University of Washington Medical Center in Seattle before training as a fellow in Medical Oncology at the Dana-Farber Cancer Institute and Harvard Medical School, where he has served as an Attending Physician since 1989.
Dr. Demetri is a Professor of Medicine at the Harvard Medical School (HMS), where he is co-Director of the Ludwig Center at Harvard and a co-director of the HMS post-graduate course entitled “High Impact Cancer Research: Cancer Biology and Therapeutics”. He also teaches a Harvard College Freshman Seminar on the Scientific, Ethical and Humanistic Aspects of Cancer. He has served on the Board of Directors of the American Association for Cancer Research (AACR) and now chairs the AACR Science Policy and Government Affairs Committee. Dr. Demetri was a founding director of the annual AACR special workshop on Translational Cancer Research for the Basic Scientist. He also serves as a member of the Board of Directors for Blueprint Medicines and Translate Bio, both in Cambridge Massachusetts.
Professor of Genitourinary Oncology, Barts Cancer Institute

Dr. Powles is a Clinical Professor of Genitourinary Oncology and the lead for solid tumor research at Barts Cancer Institute in the United Kingdom. His work focuses on a spectrum of clinical studies from Phase I to randomized Phase III, with the majority of the studies being translational Phase II investigating novel targeted and immune therapies. Alongside these trials, Dr. Powles’ research focuses on correlation of novel biomarkers and aims to define markers that are of prognostic value and can predict response or resistance to therapy. Dr. Powles has written in over 100 peer review papers in this area and has received grant income from national and international funding bodies. He graduated from St. Bartholomew’s Medical School, London in 1996, and completed his post-graduate training in oncology in 2005, receiving an MD from the University of London in 2006.
Investigator, Howard Hughes Medical Institute; Marie-Josée and Henry Kravis Chair in Human Oncology and Pathogenesis; Chairman, Human Oncology and Pathogenesis Program

Charles L. Sawyers received a BA from Princeton University in 1981 and an MD from Johns Hopkins University School of Medicine in 1985, and completed his internal medicine residency at UCSF. He became a Howard Hughes Medical Institute Investigator in 2002 while at UCLA, and then moved to the Memorial Sloan Kettering Cancer Center in 2006 where he currently serves as the Chair of the Human Oncology and Pathogenesis Program.
Sawyers studies mechanisms of cancer drug resistance with an eye toward developing novel therapies. He co-discovered the antiandrogen drug enzalutamide (Xtandi®) that was approved by the FDA in 2012 for treatment of advanced prostate cancer. He shared the 2009 Lasker-DeBakey Clinical Medical Research Award for the development of the ABL kinase inhibitor imatinib for patients with chronic myeloid leukemia and the second generation ABL inhibitor dasatinib to overcome imatinib resistance. He received the 2013 Breakthrough Prize in Life Sciences, the 2013 Taubman Prize for Excellence in Translational Medical Science and the 2015 BBVA Knowledge Award in Biomedicine.
Sawyers is a member of the National Academy of Sciences, the National Academy of Medicine (formerly IOM) and the American Academy of Arts and Sciences. He is past President of the American Association for Cancer Research (AACR) and the American Society of Clinical Investigation (ASCI), was appointed to the National Cancer Advisory Board by President Obama and has served on the Board of Directors of Novartis since 2013. He also serves as Steering Committee Chair of the AACR Project GENIE, an international consortium of cancer centers who share genomic and clinical data from patients treated at their respective clinical sites.
Director of the Center for Cellular Immunotherapies, University of Pennsylvania

Carl June is the Richard W. Vague Professor in Immunotherapy in the Department of Pathology and Laboratory Medicine. He is currently Director of the Center for Cellular Immunotherapies at the Perelman School of Medicine, and Director of the Parker Institute for Cancer Immunotherapy at the University of Pennsylvania. He is a graduate of the Naval Academy in Annapolis, and Baylor College of Medicine in Houston, 1979. He received his graduate training in immunology and malaria with Dr. Paul-Henri Lambert at the World Health Organization, Geneva, Switzerland from 1978-79, and his post-doctoral training in transplantation biology with E. Donnell Thomas and John Hansen at the Fred Hutchinson Cancer Research Center in Seattle from 1983 – 1986. He is board certified in Internal Medicine and Medical Oncology. He maintains a research laboratory that studies various mechanisms of lymphocyte activation that relate to immune tolerance and adoptive immunotherapy for cancer and chronic infection. In 2011, his research team published findings detailing a new therapy in which patients with refractory and relapsed chronic lymphocytic leukemia were treated with genetically engineered versions of their own T cells. The treatment has now been used with promising results to treat children with refractory acute lymphoblastic leukemia. He has published more than 350 manuscripts and is the recipient of numerous prizes and honors, including election to the Institute of Medicine in 2012 and the American Academy of Arts and Sciences in 2014, the William B Coley award, the Richard V Smalley Memorial Award from the Society for Immunotherapy of Cancer, the AACR-CRI Lloyd J. Old Award in Cancer Immunology, the Philadelphia Award in 2012, the Taubman Prize for Excellence in Translational Medical Science in 2014 (shared w S. Grupp, B. Levine, D. Porter), the Paul Ehrlich and Ludwig Darmstaedter Prize (shared w J. Allison), the Novartis Prize in Immunology (shared w Z. Eshaar and S. Rosenberg), the Karl Landsteiner Memorial award, the Debrecen Award and a lifetime achievement award from the Leukemia and Lymphoma Society.
Chief Scientist & Executive Partner, Flagship Pioneering
Rupert served as Executive Vice President and President, Research and Early Development for Bristol Myers Squibb (BMS). He joined BMS with its acquisition of Celgene in 2019. In this role, he led Research and Early Development, overseeing the advancement of promising programs, technologies and assets across all therapeutic areas, from discovery through proof-of-concept. He also worked closely with Business Development to identify external sources of innovation. In addition, he served as the executive sponsor of the Organization for Latino Achievement (OLA) People and Business Resource Group and the Bristol Myers Squibb STEM Council.
Rupert joined BMS from Celgene, where he served as president of the company’s Research and Early Development organization. Prior to joining Celgene in 2015, Rupert spent ten years at Merck, where he was responsible for numerous drug development programs and held leadership roles in the company’s Early Development, Discovery Sciences, Drug Discovery and Informatics groups, as well as its respiratory and immunology franchise. Prior to his time at Merck, he spent five years at GlaxoSmithKline involved in drug discovery, experimental medicine and early clinical development of therapeutics for respiratory and immune diseases.
Rupert graduated from Oxford University in the United Kingdom with degrees in physiological sciences (MA), clinical medicine (BM, BCh) and a doctor of philosophy (DPhil) in molecular immunology. He is an elected fellow of the Royal College of Physicians.
CellCarta has a deep understanding of the regulatory requirements critical to the success of its partners and is committed to providing the highest quality every step of the way.
We were inspected and accredited for specific CAP and CLIA tests by the College of American Pathologists. We also welcome and host multiple sponsor audits to ensure sponsor requirements are met and opportunities for improvement are identified.
Careers
If you are passionate about science, join CellCarta and help shape the future of precision medicine.
