CellCarta's High-level Quality Management Systems for all Activities of Laboratory Services
Introducing CellCarta's Quality Management Systems (QMS)
In accordance with applicable GCP/GLP/GCLP regulations, our quality management program is implemented at all levels of the organization where studies are planned, conducted, monitored, reported, and archived.
We are accredited by CAP and have CLIA certifications for specific testing methods and are committed to providing the highest quality in the execution of all projects.
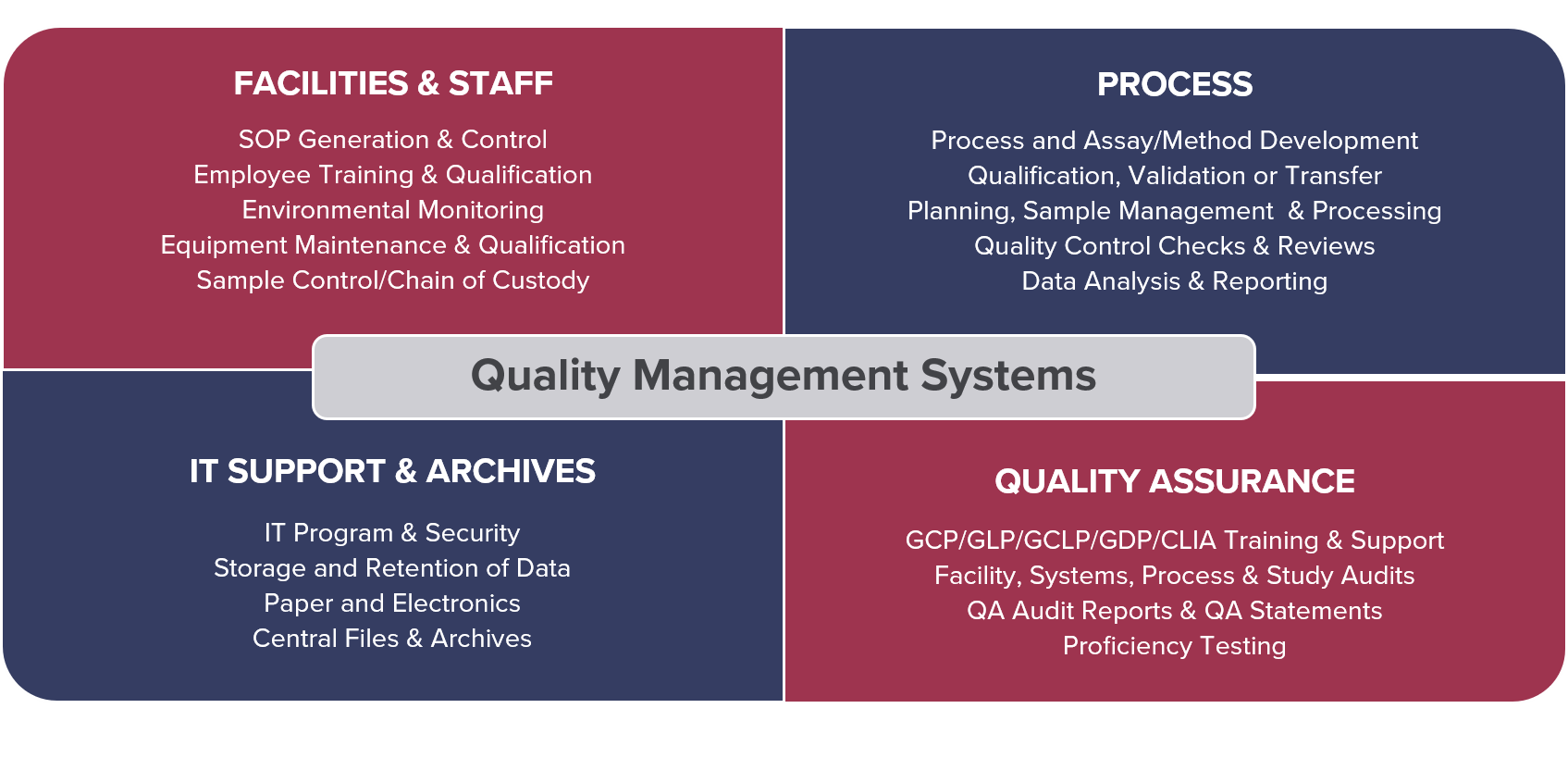
Our overarching Quality Management Systems (QMS) encompass the organization and facilities, safety and personnel, computer systems and equipment, sample management and chain of custody, assay/method qualification and validation, study planning, sample processing and data analysis, reporting, document control, storage and retention of data.
Processes and Procedures are performed by highly qualified and trained personnel and recorded in controlled documents such as study work plans, Standard Operating Procedures (SOP), Client Specific Procedures (CSP), and study worksheets/forms. All documents are approved prior to use.
Analytical Assays that meet the applicable regulatory requirements are developed, qualified and validated under our Quality Management Systems.
Data Reporting, including format and method, is developed in close interaction with the sponsor to ensure the effective communication of test results that meet sponsor’s requirements.
Quality Control (QC) is performed and documented throughout the execution of laboratory testing to ensure reliable results.
Quality Assurance (QA) audits performed by the Quality Assurance Unit are in accordance with GLP/GCP regulatory requirements, study plans, and applicable procedures. QA audits measure and monitor the performance of laboratory services and identify opportunities for improvement.
Proficiency Testing allows for the standardization of selected assays among laboratories, as required by the CAP accreditation and CLIA certification.