Accelerate the approval of your cell therapy with precision biomarker support
Rapid cell therapy development is challenging
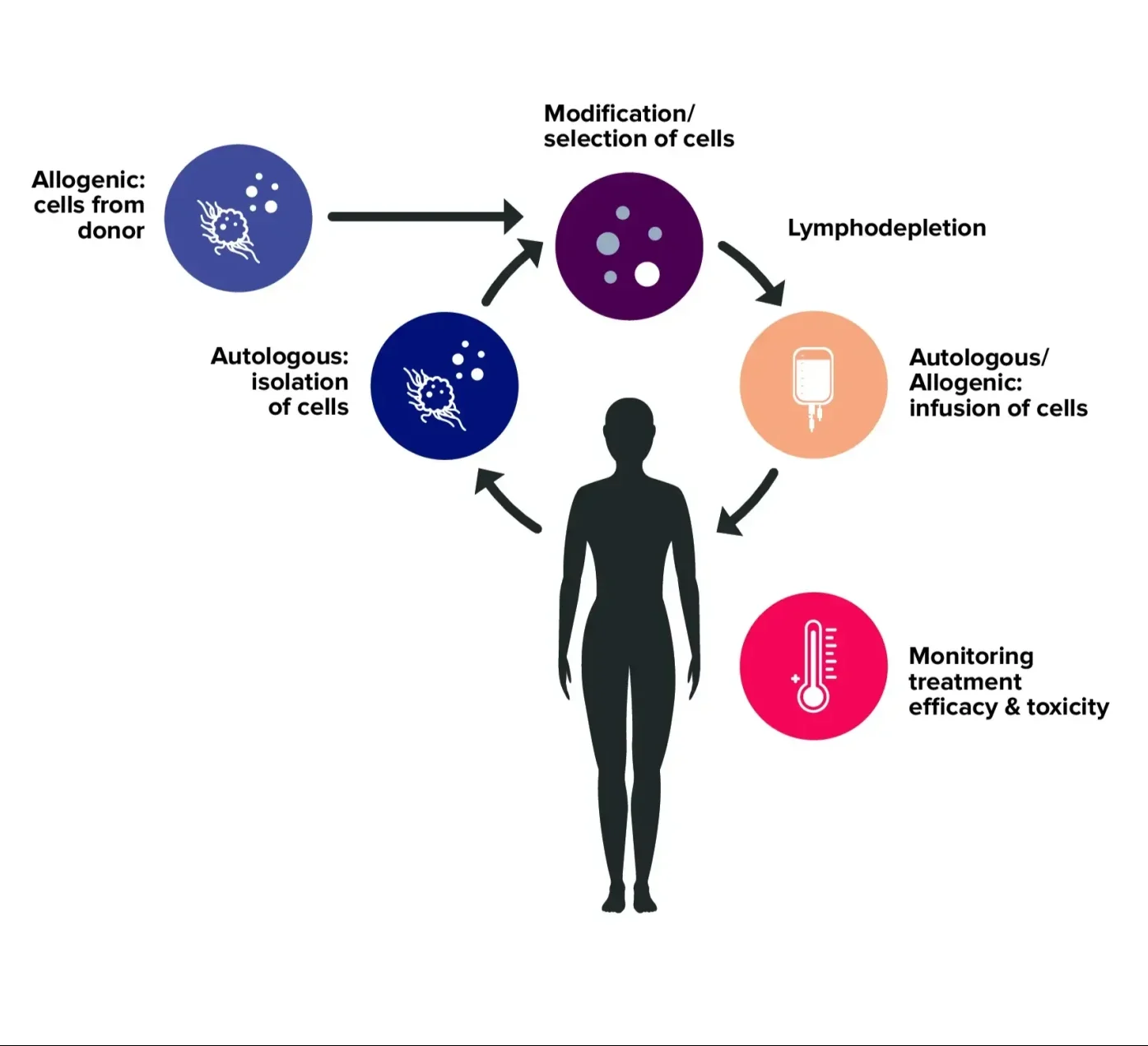
Cell therapy holds exceptional promise in healthcare, as demonstrated by the FDA approval of CAR-T and tumor-infiltrating lymphocytes (TIL) therapies. The field continues to grow rapidly, with powerful third and fourth generation CAR-T treatments, in vivo CAR generation, and the repurposing of anti-CD19 CAR-T cells for autoimmune indications.
However, cell therapies are challenging to develop and test, particularly as their complexity increases with each generation.
Partnering with a global cell therapy CRO like CellCarta provides expert guidance through each development phase- from preclinical characterization to clinical endpoint measurement.
To ensure success, developers must:
- Undertake thorough product characterization during development
- Select the most appropriate patients and assess their immune and genomic profile before treatment
- Monitor both the product and the patient’s response following treatment
To accelerate cell therapy development and approval, you need a CRO that offers comprehensive solutions, anticipates clinical testing challenges, and provides thorough sample logistics services, all supported by deep scientific expertise.
Contact our experts to see which services you need to drive your program forward.
How can CellCarta accelerate cell therapy development?
Obtain the quality data needed to move your cell therapy program forward by partnering with CellCarta. Benefit from our:
- Expertise: Over 50 completed and ongoing projects with key pharmaceutical partners in cell therapy
- Customization: Tailored solutions unique to your cell therapy
- Rigorous standards: College of American Pathologists (CAP) accredited and Clinical Laboratory Improvement Amendment (CLIA) certified to meet primary and secondary endpoint requirements
- Extensive offerings: Data analysis, sample logistics, and many more services provided
Our role as a cell therapy services CRO includes support across clinical trial design, sample logistics, data collection and data analysis.
Enhance your product development
Get the crucial data you need to make informed decisions during clinical by working with CellCarta. Our comprehensive range of platform technologies enables us to:
- Determine vector copy number (VCN), monitor retroviruses (RCR) and/or lentiviruses (RCL), with customed validated assays for these targets, using digital PCR (dPCR) and quantitative PCR (qPCR)
- Gain insights at the cellular level, including absolute cell count and cellular phenotyping with flow cytometry
- Evaluate the drug product at the single-cell level
We also support pharmacokinetics analysis of VCNs, CAR tumor infiltration analysis
Improve your patient selection
Selecting the right patients is vital to limit toxicity and boost treatment efficacy. CellCarta has an in-house team of board-certified pathologists, helping improve your patient stratification.
Gather detailed insights to inform patient selection and pre-treatment profiling by performing:
- HLA typing using next-generation sequencing (NGS)
- Drug target evaluation (e.g. BCMA) using immunohistochemistry (IHC), qPCR, and mass spectrometry
- Lymphodepletion measurement (TBNK), immune exhaustion evaluation, and bridging therapy detection (i.e. rituximab) using flow cytometry and ELISA
- Genomic profiling of key biomarkers, the tumor mutational burden (TMB), and microsatellite instability (MSI) with qPCR, NGS, targeted RNAseq, and ISH
Our biomarker-based patient profiling supports precision therapy delivery, clinical trial eligibility screening, and responder prediction.
Thoroughly assess your therapy’s safety
Obtain the data needed for therapy approval with CellCarta’s safety assessment capabilities, including:
- RCR/RCL measurements with qPCR
- Immunogenicity (cellular immune response) determination using enzyme-linked immunosorbent spot (ELISpot) assays and flow cytometry
- Cytokine assessment to understand optimal dosing and characterize cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS) andmacrophage activation syndrome (MAS), with MesoScale Delivery (MSD)® assays, Olink®, and ELISA
Accurately determine the patient’s response
Thoroughly assess therapy efficacy over time with an expert partner. CellCarta uses extensive technologies and techniques to gather vital patient treatment response data:
- Immune profiling and TBNK with flow cytometry, MSD®, Olink® and ELISA
- Assessment of tumour burden through circulating tumour cells (CTC) and ctDNA tracking using RareCyte®, NGS, and qPCR
- Tumor status measured with qPCR, IHC, NGS
- Key soluble targets (i.e. BCMA) measured using mass spectrometry, MSD® , and ELISA
Carefully monitor your drug product
Monitor your drug product with:
- Pharmacokinetics (PK) analysis of VCN using dPCR
- Cellular kinetics through CAR enumeration by flow cytometry
- CAR tumor infiltration analysis with IHC/multiplex immunofluorescence (mIF)