IHC and IF Assays for Robust Tissue Biomarker Strategies
Introducing High-quality IHC and IF assays for your clinical trials
CellCarta has developed and validated over 200 assays which have been tested and used in numerous exploratory projects and clinical trials, including global Phase III projects.
Our team of board-certified pathologists, deeply experienced imaging scientists, and laboratory technologists have developed a proven process for IHC and IF assay development which is embedded into our quality systems.
We provide customizable IHC and IF assay development
We provide complete IHC and IF assay development services, from:
- the selection of high-quality antibodies to
- proper selection of platforms
- control tissues, and
- experimental conditions
to obtain the best signals.
At CellCarta, we adopt a full accountability philosophy for our assay development projects.
While we select high-quality antibody vendors, CellCarta has established SOPs guiding the quality of the reagents, including detecting variations from reagent lots.
Our approach to assay development is customizable and optimized to your needs and validation requirements.
A complete validation report including assay precision, specificity, and performance can be provided and the final protocol is locked and described in an SOP.
Automated platforms for IHC and IF assays
CellCarta automates its IHC and IF assays to minimize variability and increase productivity and capacity.
The following automated staining platforms are currently available.
- Dako Autostainer Link48 (+ PT-modules)
- Dako Omnis
- Ventana Benchmark Ultra
- Ventana Discovery Ultra
- Leica Bond
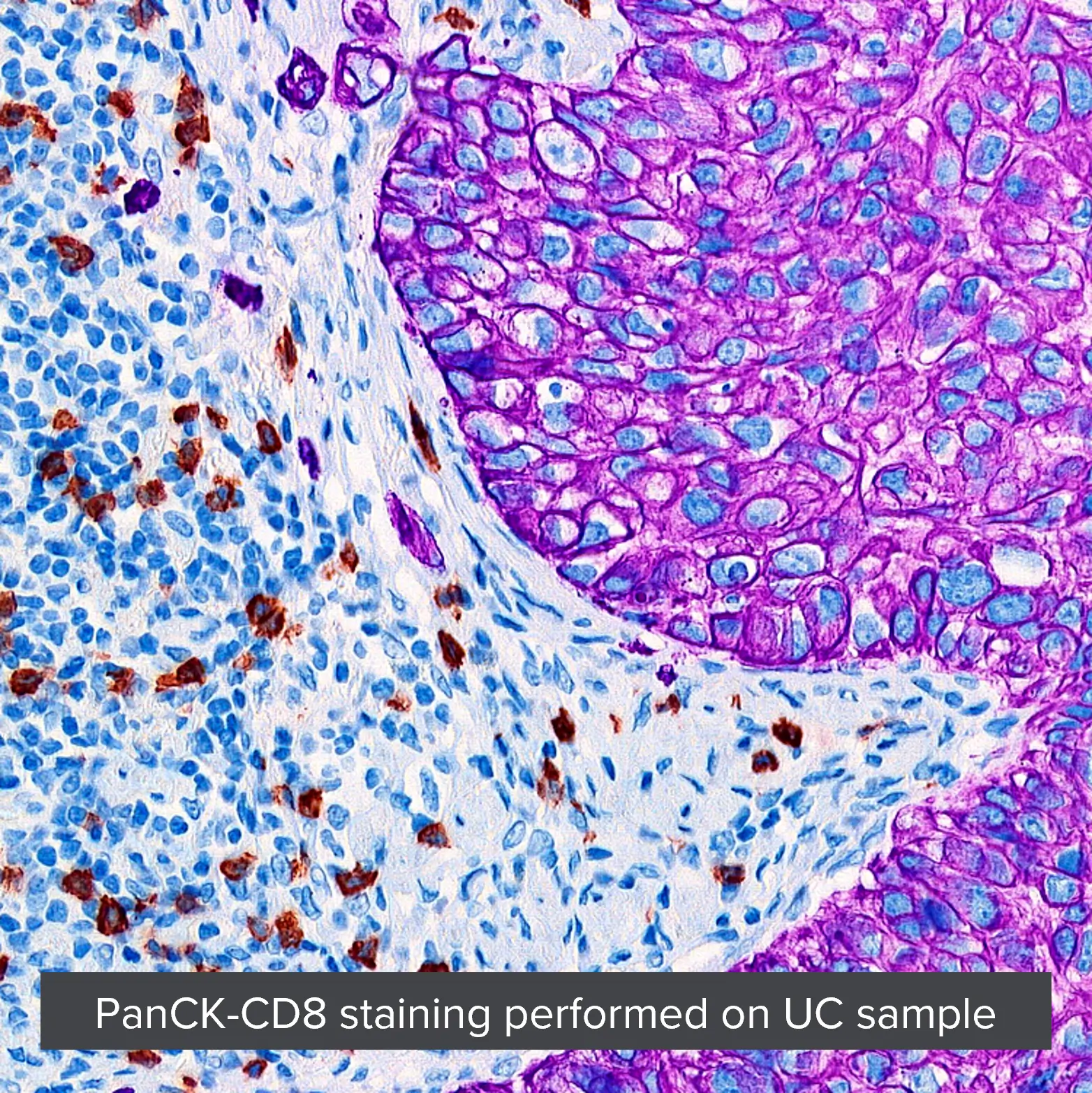
Introducing Multiplex IHC and Multiplex IF ((mIHC and mIF)
With multiplexed IHC, you interrogate multiple targets on a single slide, obtaining
- quantitative and actionable results
- and leveraging often limited tissue specimens.
Multiplex IF offers a solution for the study of multiple targets on a single slide, where the targets are expressed on the same cellular compartment.
mIHC and mIF biomarker assays: advantages of our standardized workflow
- Maximum use of scarce or minimally available tissue specimens
- Single view, multianalyte information integration for cohesive understanding of mechanistic pathways
- Increased visualization of multianalyte intra-tumor (tumor microenvironment and tumor regions) response(s) to candidate therapeutic
- Detailed capturing of cellular infiltrations and associated activation status
- Development of panel-specific mIHC and mIF biomarker assays e.g. for assessing immune response to support immunotherapy drug development
- Quantitative data generated by trained pathologists supported by AI platforms