Powering up your CDx Development: expert support by CellCarta every step of the way
Why develop an IVD CDx?
Therapeutic drug clinical trials that require appropriate patient selection as part of the inclusion/exclusion criteria may need an investigational in vitro diagnostic (IVD) medical device for medical management decisions.
A companion diagnostic (CDx) is an in vitro diagnostic device that provides information essential for the safe and effective use of the corresponding therapeutic product.
With a CDx, you can identify which patients are most likely to benefit from therapy, thus reducing the risk of side effects and improving overall patient outcomes.
As a leading provider of precision medicine solutions, we offer expertise in assay development and validation to guide the transition of your assay from investigational clinical trial assay to IVD CDx.
Our services are provided for a broad range of platforms: next-generation sequencing (NGS), PCR, immunohistochemistry (IHC), in situ hybridization (ISH), and fluorescence ISH (FISH).
How does CellCarta support IVD CDx development?
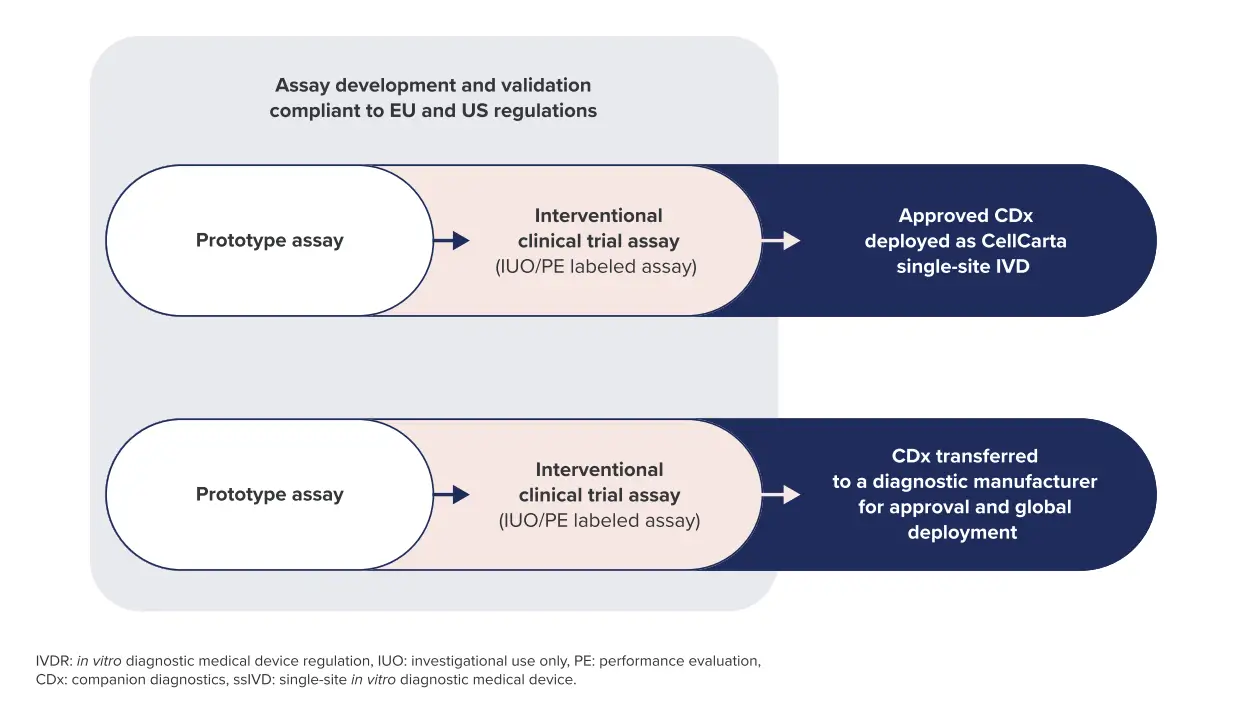
CellCarta can support taking your assays from prototype assay to IVD CDx with quality management systems and a team of regulatory experts to ease your submission process.
With our ISO13485 framework, we design and develop assays for use in patient-interventional studies. Your interventional clinical trial assay is developed and validated to be compliant with EU and/or US regulations, including IVD regulation (IVDR).
Once approved, a CDx can be deployed as a single-site IVD (ssIVD) at one of our laboratories with the possibility of transfer to distributed IVD manufacturer if needed.
Our team also runs analytical and clinical performance studies following US FDA and EU (IVDR) requirements.
CellCarta’s regulatory capabilities
CellCarta provides extensive regulatory support to facilitate performance study submissions across major complex markets such as the EU and US.
- FDA study risk determination
- IRB and ethics committee submissions
- IVDR performance evaluation (Annex XIII, and XIV)
- Support on local country requirements such as HGRAC submission in China
Our knowledgeable team adapts to the dynamic regulatory landscape, including the shift from IVD Directive (IVDD) to IVD Regulation (IVDR), making us a reliable partner.