Gain Biotherapeutic Insights with Receptor Occupancy Assays via Flow Cytometry
The Role of Receptor Occupancy Assays in Drug Development
As precision medicine progresses at an accelerated pace, understanding how biotherapeutics engage their targets is crucial to moving forward confidently in the drug development process.
RO assays are frequently used to generate pharmacodynamic (PD) biomarker data which can be coupled to pharmacokinetic (PK) profiles to model PK/PD relationships.
Their applications in pre-clinical and early clinical stages are wide and include assisting in
- compound screening
- demonstrating target engagement in disease models
- and determining the starting dose for future use in trials.
In later stages of drug development, RO assays are used in combination with PK profile to inform decisions on dose selection and administration schedules.
Using the power and flexibility of CellCarta’s flow cytometry platform, we can measure receptor binding to ultimately understand biotherapeutics PK/PD.
At CellCarta, we ensure that the results generated from our RO assays are reliable and of the highest quality.
Navigating RO Assays: trust CellCarta for basic to advanced solutions
Our team of experienced scientists can develop custom RO assays from the ground up or transfer already designed assays to our labs.
When entrusting us to develop or run fit-for-purpose RO assays, expect the following:
- Simultaneous detection of RO and other PD markers using multiparametric flow cytometry
- Reagent specificity determined by extensive screening during assay development
- Optimal matrix selection: whole blood or PBMC
- Rigorous quality control in accordance with relevant regulatory requirements, including GCLP
Customizing Receptor Occupancy Assays to your objectives
Different approaches can be taken to investigate the binding capabilities of biotherapeutics depending on the reagents available.
At CellCarta, we have capabilities to investigate them all.
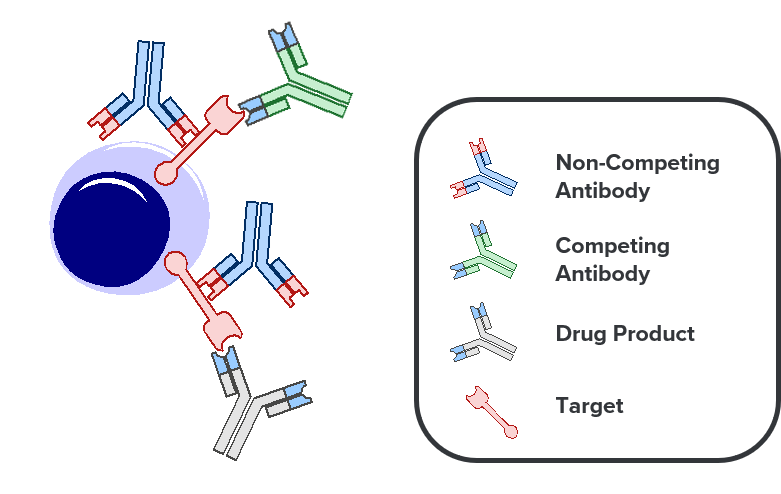
Competing vs non-competing antibodies:
Competing and non-competing antibodies are added to the whole blood sample of a subject previously infused or not with the drug product.
Competing antibodies will bind the target where the drug product normally binds, providing the number of targets not currently bound by the drug.
The non-competing antibodies will bind the target on a different site, providing the number of total available targets for the drug.
The ratio of the competing vs non-competing results provides the level of RO of the drug
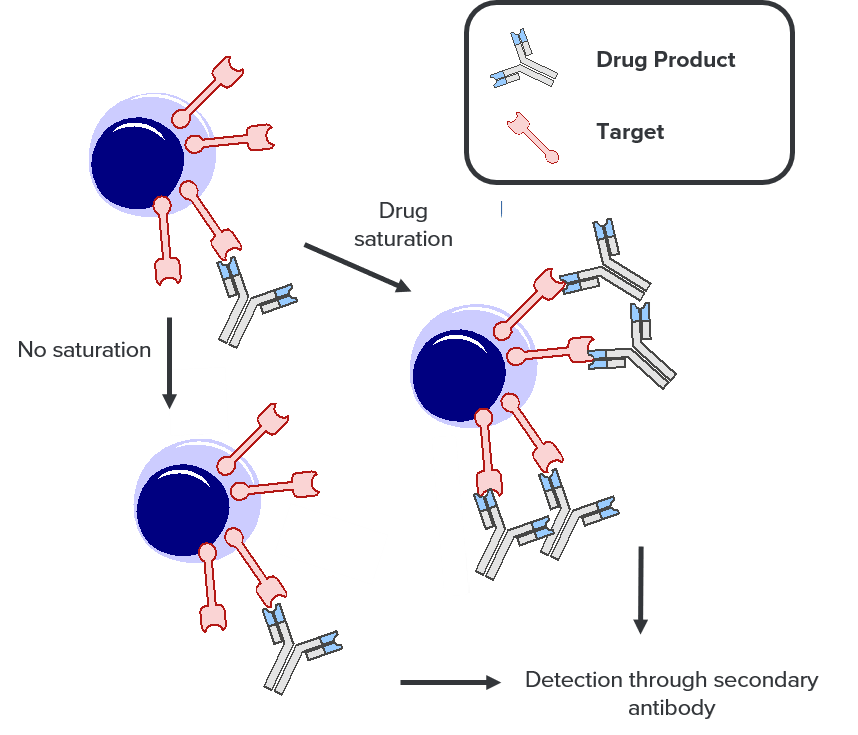
Saturated vs non-saturated with direct detection of the drug:
Half of the sample is saturated with the drug product, ensuring that all available target sites are bound by the drug, providing the number of total available targets.
The other half is not saturated, reflecting how the target is bound by the drug product in the patient.
The drug product is then detected through a secondary antibody staining and the ratio of the “unsaturated” sample over the “saturated” provides the level of RO of the drug product.
This assay is particularly useful when monoclonal antibodies to the receptor are not available.
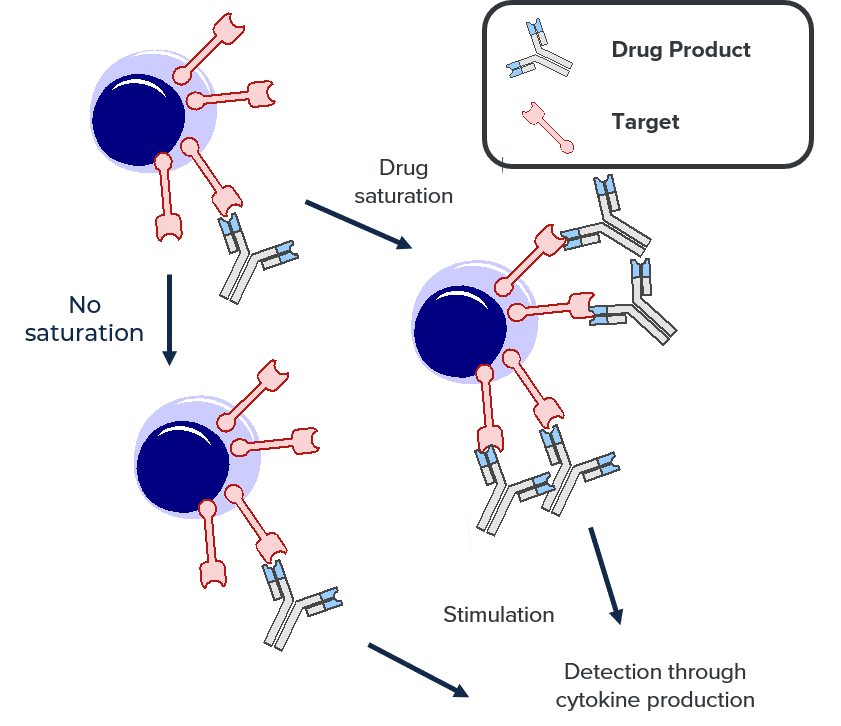
Saturated vs. non-saturated with a functional readout:
This assay follows the same methodology as the saturated vs. non-saturated method described above, except that function is investigated as a correlate of the level of RO instead of direct detection of the bound drug product.
Half of the sample is saturated with the drug product and the other half is saturated with an isotypic control.
Following an in vitro stimulation, the levels of cytokine(s) production are monitored from both conditions. The ratio of both levels of cytokine production provides the RO of the drug product.
This assay is ideal for the measurement of RO of bi-modal antibodies.