August 18, 2025
Patient enrollment: a clinical trial bottleneck
Patient enrollment can be a significant challenge in clinical trials, particularly when it comes to identifying the right patients for trial recruitment. Globally, more than 80% of clinical trials fail to meet required enrollment numbers on time, often resulting in costly study extensions or the addition of new trial sites.1
In precision oncology, tight timelines, limited patient pools, and narrow eligibility criteria complicate trial patient selection, often creating delays that stall promising therapies and escalate development costs. Finding an efficient way to identify the most suitable patients is essential to ensure smooth trial operations and accurately identify a drug’s clinical benefits.
Why identifying driver mutations matters
One of the most effective ways to improve patient enrollment efficiency is to select patients based on the molecular features most relevant to the treatment being studied. In oncology, this involves carrying out tumor mutation profiling.
Depending on the study goals, profiling might involve targeted sequencing of key oncogenes such as EGFR, KRAS, FGFR, BRAF, or PIK3CA, or broader panels that detect co-occurring mutations, gene fusions, resistance mechanisms, or biomarkers like microsatellite instability (MSI), and tumor mutational burden (TMB).2
Although molecular profiling has become a standard tool in oncology care (helping clinicians match patients to targeted therapies based on their tumor biology), in early exploratory research and clinical trials, it is often underused. As a result, many studies still rely on broader selection criteria, making it harder to recruit the right patients.
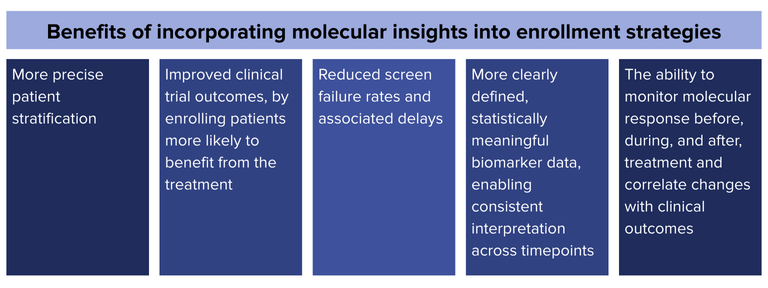
Incorporating molecular insights into enrollment strategies enables more precise patient stratification, allowing for:
- Improved clinical trial outcomes, by enrolling patients more likely to benefit from the treatment
- Reduced screen failure rates and associated delays
- More clearly defined, statistically meaningful biomarker data, enabling consistent interpretation across timepoints
- The ability to monitor molecular response before, during, and after, treatment and correlate changes with clinical outcomes
While many sponsors recognize the importance of this approach, it can be challenging to determine which molecular profiling assay is best suited to a clinical trial’s specific needs, as assays must strike the right balance between scientific depth and operational practicality. With broad tumor profiling assays, the choice is easier. Because they are already validated and designed to include clinically relevant genes, they offer a practical, ready-to-implement option that can be integrated into patient enrollment without adding unnecessary complexity.
Contact us to find out how we can support with tumor mutation profiling
Fast, ready-to-deploy assays
To support streamlined patient selection and monitoring in clinical trials, CellCarta offers a wide portfolio of genomic assays, including three broad, validated tumor profiling panels that identify key driver mutations and clinically relevant gene expression. These assays deliver both broad and targeted profiling options and are ready to implement for clinical use, enabling rapid deployment for patient enrollment.
For studies requiring a more tailored approach, CellCarta also offers custom panel development.
oncoReveal® CDx: NGS based CDx test for key oncogenes
oncoReveal® CDx is an IVDR and FDA approved, next-generation sequencing (NGS)-based companion diagnostic (CDx) test, developed to provide rapid, clinically actionable insights across a wide range of solid tumors.
A streamlined single-tube workflow and high sensitivity enables fast turnaround and reliable performance, even on low DNA input clinical samples.
- Gene coverage: 22 clinically relevant genes, including EGFR, KRAS, BRAF, PIK3CA
- Tumor types: non-small cell lung cancer (NSCLC), colorectal cancer (CRC), and pan-cancer solid tumor
- Sample type: DNA from formalin-fixed paraffin-embedded (FFPE) tissue samples
- Sensitivity: detects CDx variants down to 1.5% variant allele frequency (VAF), and non-CDx tumor profiling variants down to 1.4-2.2% VAF
CellCarta is the first CRO to offer the oncoReveal® CDx pan-cancer panel to support patient management in clinical studies.
TSO500 Comp: Pan-cancer NGS assay for DNA and RNA variants
For trials that require broader genomic coverage, we also offer the The TruSight Oncology 500 (TSO500) panel. TSO500 Comp is a comprehensive pan-cancer NGS panel enabling simultaneous analysis of DNA and RNA variants across hundreds of genes, making it well-suited for exploring complex molecular signatures, co-occurring alterations, and emerging biomarkers.
- Gene coverage: 523 pan-cancer genes for DNA variants, 55 for RNA
- Tumor types: a broad range of solid tumor types, including breast, colorectal, lung, and ovarian
- Sample type: DNA and RNA from FFPE tissue samples, and blood-derived ctDNA
- Sensitivity: ≥ 95% (small variants, 5% VAF)
Aspyre® Lung: Ultra-sensitive detection of NSCLC biomarkers
Aspyre® Lung is a clinically validated qPCR-based assay enabling ultra-sensitive mutation detection across NSCLC genes, with a rapid turnaround time and low sample input requirements.
- Gene coverage: 11 NSCLC genes; 77 variants for DNA (including EGFR, BRAF, KRAS, and ERB2), and 36 for RNA (including ALK, ROS1, MET, and NTRK1)
- Tumor type: Non-small cell lung cancer
- Sample type: FFPE-derived DNA and RNA, and blood-derived cfDNA and cfRNA
- Sensitivity: ≤ 3% VAF (tissue) or3-0.8% VAF (Blood)
Case study: supporting patient selection where standard assays fall short
CellCarta collaborated with a large global biopharma company to support patient enrollment in a study of high-risk non–muscle-invasive bladder cancer (HR-NMIBC), where no standard NGS assay was available. Working alongside Pillar Biosciences, the team rapidly implemented and validated a customized solution by combining two existing targeted NGS panels, and clinical samples from the CellCarta biobank.
The two panels, OncoReveal™ Essentials LBx and Fusion LBx, covered key DNA mutations and RNA fusions, including FGFR alterations relevant to the study population.
The customized approach enabled accurate, sensitive detection from limited samples, allowing the sponsor to shift from an existing qPCR assay to an NGS-based strategy that better suited their enrollment goal.
Access fast, reliable, consistent profiling
CellCarta works with clinical trial teams to help make tumor mutation profiling fast and easy to implement, and more reliable across sites. We offer:
- Ready-to-deploy, off-the-shelf assay options, as well as customized assay options, depending on your biomarker strategy
- Expertise in all and access to all other major platforms, enabling flexibility across study designs
- Global Reach with genomics labs in China, Europe, North America, all with standardized SOPs for coordinated operations across trial sites
- Expert support for challenging samples, enabled by in-house pre-analytical services
Interested in how our tumor mutation profiling services could support your next trial? Contact us to speak to one of our experts
You might also be interested by
CellTalk Blog
Improving RNA-Seq Library Preparation with the Watchmaker Workflow
November 5, 2025
Genomics
More infoPosters
A Quantitative Mass Spectrometry Workflow for Highly Multiplexed Measurement of Immunomodulatory Proteins to Support Immunotherapy Clinical Trials
October 13, 2023
Genomics
More infoCase Studies & Whitepapers
Accurately Predict Immune Checkpoint Inhibition Response Using Only RNA-Seq Data
June 27, 2023
Genomics
More info