August 18, 2025
The risk of poor target selection in preclinical development
In early-stage drug development, there’s often a rush to move promising candidates forward. Tight timelines and competitive pressures can lead to companies having an over-reliance on ‘quantity over quality’ in research and development,1 hoping to maximize their chances of success by bringing more products into the pipeline.
While this may offer short-term momentum, advancing drug candidates without establishing a strong link between target and disease at the preclinical stages can lead to costly, later-stage setbacks. When targets are poorly defined, programs risk progressing on shaky foundations that can compromise other aspects of the pipeline, from study design and patient selection to commercial potential.
Understanding what makes a target worth pursuing, and how to evaluate it effectively, is key to laying the groundwork for clinical success.
Improving trial outcomes starts with the right target
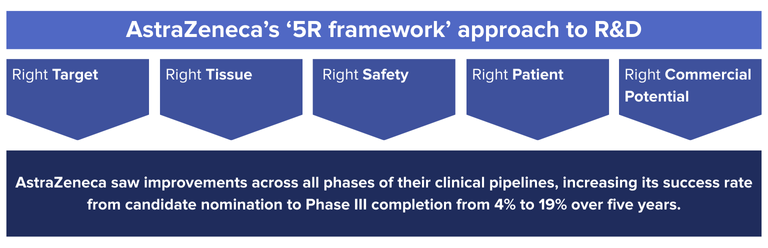
Developing a strong biological foundation for your target is a key driver in the downstream success of a therapeutic candidate. One example of this is AstraZeneca’s ‘5R framework’ approach to R&D, in which they aimed to improve their R&D productivity by focusing on 5 technical determinants: the right target, right tissue, right safety, right patient, and right commercial potential. 1
The ‘right target’ principle emphasized selecting targets with a strong link to disease, with a focus on developing a deeper initial biological understanding through fewer, more informative screens—using technologies such as high-content imaging, high-throughput electrophysiology, and advances in genomics technologies such as transcriptomics.
The result? AstraZeneca saw improvements across all phases of their clinical pipelines, increasing its success rate from candidate nomination to Phase III completion from 4% to 19% over five years.1 By focusing only on the most promising candidates and providing robust study data to inform late-stage clinical trials, not only did they improve candidate success rate, they also saved research costs and accelerated all phases of their clinical trials.
This improvement is a clear demonstration of the impact that strong preclinical research can have on downstream outcomes. A greater focus on target quality in early development enables teams to prioritize candidates with stronger potential for therapeutic impact, increasing the likelihood of success.
The key role of genomics in better target selection
Genomics is a key tool in enabling better target selection, allowing researchers to understand the biological relevance of a target at a molecular level. The growth of techniques in next-generation sequencing (NGS ), RNA sequencing, whole exome sequencing (WES), single-cell sequencing, T-cell/B-cell receptor (TCR/BCR) profiling, and associated analytical methods refining mutation profiling allows teams to uncover associations between genes, pathways, and disease, and assess whether modulating a particular target will likely yield clinical benefit.
In preclinical research, these insights are especially valuable when deciding which candidates to advance, enabling:
- Identification of genetically validated targets with known links to human disease
- Clarification of the mechanism of action (MOA) by showing how a compound influences gene expression or pathway activation
- Detection of early biomarkers that provide later value in patient stratification or response monitoring
- Identification of potential off-target effects or resistance mechanisms that could impact long-term viability
Get in touch to find out how we can support your preclinical development.
The impact of early genomic insight on downstream success
The value of genomic profiling doesn’t end in early development. When applied strategically, molecular insights generated in preclinical research can guide key decisions throughout the pipeline.
Early identification of response biomarkers can support more focused trial designs, while understanding resistance pathways can shape monitoring strategies and combination therapy planning. Early-stage genomics work can also lay the foundation for companion diagnostics, enabling the targeted therapy to launch with a clearly defined patient population.
Advances in NGS have led to further investment in genomics to help identify novel, genetically validated targets in early development.1 As such, NGS is now more accessible in early development, allowing teams to build a stronger understanding of target biology and downstream biomarker opportunities sooner in the process.
Case study: Osimertinib and the value of a well-characterized target
The development of Osimertinib, a treatment for non-small cell lung cancer (NSCLC), illustrates how genomic insight can strengthen target confidence and accelerate clinical development.1 In NSCLC, the EGFR T790M mutation had been identified as a common resistance mechanism in patients progressing on first-generation EGFR inhibitors.
AstraZeneca recognized the clinical relevance of this mutation, designing Osimertinib to address this specific, established resistance mechanism. Because the EGFR T790M target was well-characterized, the AstraZeneca team were able to design a more tailored clinical program and use a mutation-specific companion diagnostic for more precise patient selection from the outset.
Combining this with the other elements of the 5R framework resulted in Osimertinib having one of the fastest recorded clinical development programs, going from initial human dosing to launch in just over 2.5 years. The program’s success is a clear example of how well-validated genomic targets can inform trial design, enable targeted patient selection, and accelerate path to market.
Support early development decisions with the right tools and expertise
Translating early genomic insight into clinical success requires the right assays and dedicated expertise. CellCarta offers a wide range of tools suited to preclinical and translational research, including RNAseq, whole genome and whole exome sequencing, single-cell analysis, and Olink proteomics. For companies that require a tailored approach, we offer custom assay development, alongside several validated panels designed around well-established genes, which can streamline trial efficiency. Our validated assay offerings include:
- oncoReveal® CDx: An FDA-approved targeted NGS panel covering 22 key genes across numerous tumor types
- TSO500: A comprehensive pan-cancer panel covering 523 genes for DNA variants and 55 for RNA variants
- Aspyre® lung: Ultra-sensitive, high-fidelity multiplex PCR/reverse-transcriptase (RT)-PCR, covering 11 genes and including a fusion panel for the detection of established NSCLC biomarkers in both DNA and RNA.
By combining these advanced validated platforms with custom analysis pipelines, we can help sponsors narrow down the most promising biomarkers and transition seamlessly into focused platforms, such as qPCR, dPCR, or NGS, at the later clinical trial stages.
Our capabilities can support both small and large pharma companies with early-phase development, right through to regulatory submission. Combined with our global infrastructure, expertise in handling challenging samples, and access to all major genomic analysis platforms, CellCarta ensures high-quality data from early discovery through clinical execution, aiding success at all stages of the pipeline.
Want to learn more about how our services can support your preclinical research? Chat with one of our experts!
References
You might also be interested by
CellTalk Blog
Immortalizing Your Samples: The Power of Dual Extraction in Pre-Analytical Planning
August 18, 2025
Genomics
More infoCellTalk Blog
Maximize Sample Value with Combined IHC and RNAseq Analysis
August 18, 2025
Genomics
More infoWeb News
Biofidelity and CellCarta partner to deploy Aspyre® Lung in global clinical trials
January 9, 2025
Genomics
More infoCellTalk Blog
How can HLA typing drive better immunotherapy development and selection?
December 6, 2023
Genomics
More info