September 19, 2025

B cells are central to both protective immunity and autoimmune pathology. With the recent shift of cell therapies from oncology indications to autoimmune diseases, interest has been growing towards this immune cell population.1,2
Flow cytometry remains the gold standard for monitoring B cells in both research and clinical settings. However, lack of convention for classification as well as reliable and stable markers, especially following thawing of samples, can make it difficult to capture rare B-cell populations relevant to autoimmune drug development.
For translational research teams, this creates an urgent need for tools that deliver the sensitivity and consistency required to fully understand B-cell dynamics and drive the development of new B-cell therapies.
The Challenge of Defining B-cell Subsets
B cells are far from uniform. Transitional, naïve, memory, double-negative, and plasma cell subsets each play distinct roles in health and disease, but their phenotypic profiles often overlap. Inconsistent marker usage and variability in gating strategies have made it difficult to classify B-cell subsets consistently, which can complicate the comparison of results across studies.3
For drug developers, the lack of standardization creates real-world hurdles. Autoimmune therapies that aim to induce a B-cell reset (eliminating autoreactive populations and allowing the immune system to repopulate with naïve B cells) depend on precise monitoring of which subsets return after the B-cell aplasia phase. Without consistent, high-resolution phenotyping, it is difficult to determine whether a therapy is resetting B-cell populations in a way that supports durable disease control.
Recent efforts to harmonize approaches are beginning to address these issues, with publications recommending standardized marker sets to improve reproducibility and resolution of B-cell subsets.³ Markers such as CD21, once regarded mainly for their functional role, are now recognized as important phenotypic indicators that help distinguish distinct populations. Incorporating markers like these into flow cytometry panels is essential to provide the clarity and consistency needed for research on B-cell reset in autoimmune diseases.
A Refined Flow Cytometry Assay for B-Cell Subsets
To address these challenges, CellCarta has advanced its flow cytometry capabilities with a refined B-cell assay that improves the sensitivity and consistency of B-cell subset profiling.
1- Updated gating strategy
We refined our panel strategy by incorporating markers such as CD21 as phenotypic indicators alongside other established markers. This enables further stratification of B-cell subtypes, providing better insight into both activated and developmentally distinct populations that are increasingly linked to autoimmune disease.
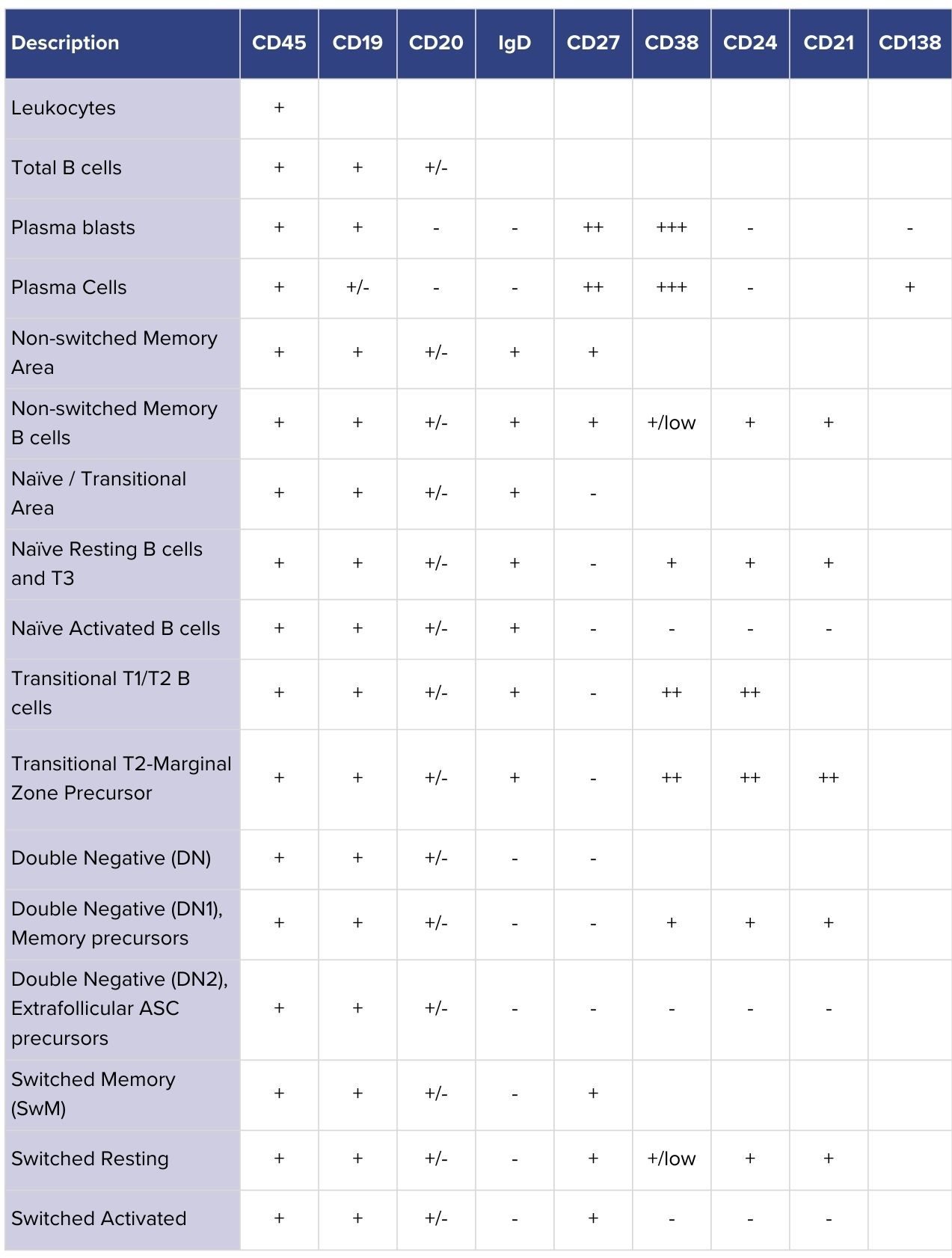
Table 1: B-cell subsets identifiable with CellCarta’s refined flow cytometry panel. Rows show major populations, while columns list the markers used to classify them. CD21 provides added resolution by distinguishing activated and developmentally distinct subsets relevant to autoimmune disease.
2- Improved bulk lysis method
We’ve developed a refined bulk lysis method using the B-cell panel. In comparison to the direct whole blood (WB) analysis (100ul of blood), the improved bulk lysis method resulted in a 10-fold increase in the cell input compared to the traditional analysis using WB for flow cytometry assay (Figure 1). As more cells are acquired, rare populations can now be assessed with better precision. The whole method was evaluated at both 4°C and room temperature (RT), providing flexibility for the sample management and transportation.
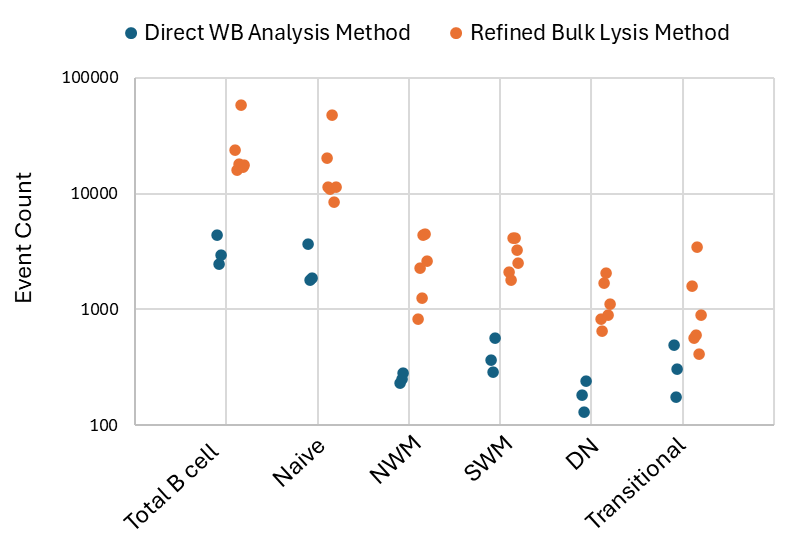
Figure 1: Event counts of total B cells and subsets acquired from healthy donors using direct whole blood analysis method (blue) and refined bulk lysis method (orange) WB – Whole Blood; NWM – Non-switched memory; SWM – Switched memory, DN – Double negative
Global Scalability With the Lyric Platform
To offer global site-to-site consistency, we switched our B-cell assay from the Fortessa to the Lyric platform, leveraging the built-in capabilities of this platform to achieve high inter-instrument/inter-site standardization. Unified workflows and directly comparable data make it easier to scale studies internationally, reduce variability, and build confidence in results across multicenter trials.
Supporting the Next Generation of B-Cell Therapies
With refinements to its gating strategy, bulk lysis method, and global platform standardization, CellCarta’s B-cell flow cytometry assay provides the resolution, sensitivity, and reproducibility needed to study B-cell dynamics in detail. In the context of the development of B-cell reset therapies, these advancements enable the precise monitoring and consistency needed to better define which B-cell populations are depleted, which repopulate, and how these shifts influence autoimmune disease outcomes.
In addition to our B-cell panel, CellCarta can support researchers with both off-the-shelf panels for rapid deployment and custom panel development tailored to specific scientific questions. With this flexibility, pharma teams can choose the right approach for their needs, supporting clearer decision-making in the development of new B–cell–targeted therapies.
References
- Lee, D. S., Rojas, O. L., & Gommerman, J. L. (2021). Nature reviews Drug discovery, 20(3), 179-199.
- Harrison C. Nature Biotechnology, vol. 42, 2024, pp. 995–997.
- Sanz, I., Wei, C., Jenks, S. A. et al (2019). Frontiers in immunology, 10, 2458.
Meet our expert:

Alex Guo, PhD, is a Principal Scientist at CellCarta, specializing in immune monitoring and proteomics. With a doctorate in Immunology and Molecular Oncology, he has extensive expertise in developing and validating novel assays to address complex clinical needs. Alex has led immune monitoring analyses across multiple clinical programs, translating high-dimensional data into actionable insights for clients. He combines deep scientific expertise with practical project execution to advance translational and clinical research.
You might also be interested by
CellTalk Blog
Flow Cytometry Applications: Advanced Uses, Benefits, and Limitations
October 17, 2024
Flow Cytometry
More infoCellTalk Blog
How to ensure consistent flow cytometry readouts between labs and optimize your clinical trials
September 18, 2024
Flow Cytometry
More infoCellTalk Blog
Spectral Flow and Mass Cytometry: Select the Right Platform for Your Clinical Study
July 2, 2024
Flow Cytometry
More info