As a global Contract Research Organization Laboratory (CRO) to the biopharmaceutical industry, CellCarta provides access to a broad offering of biomarker platforms and services.
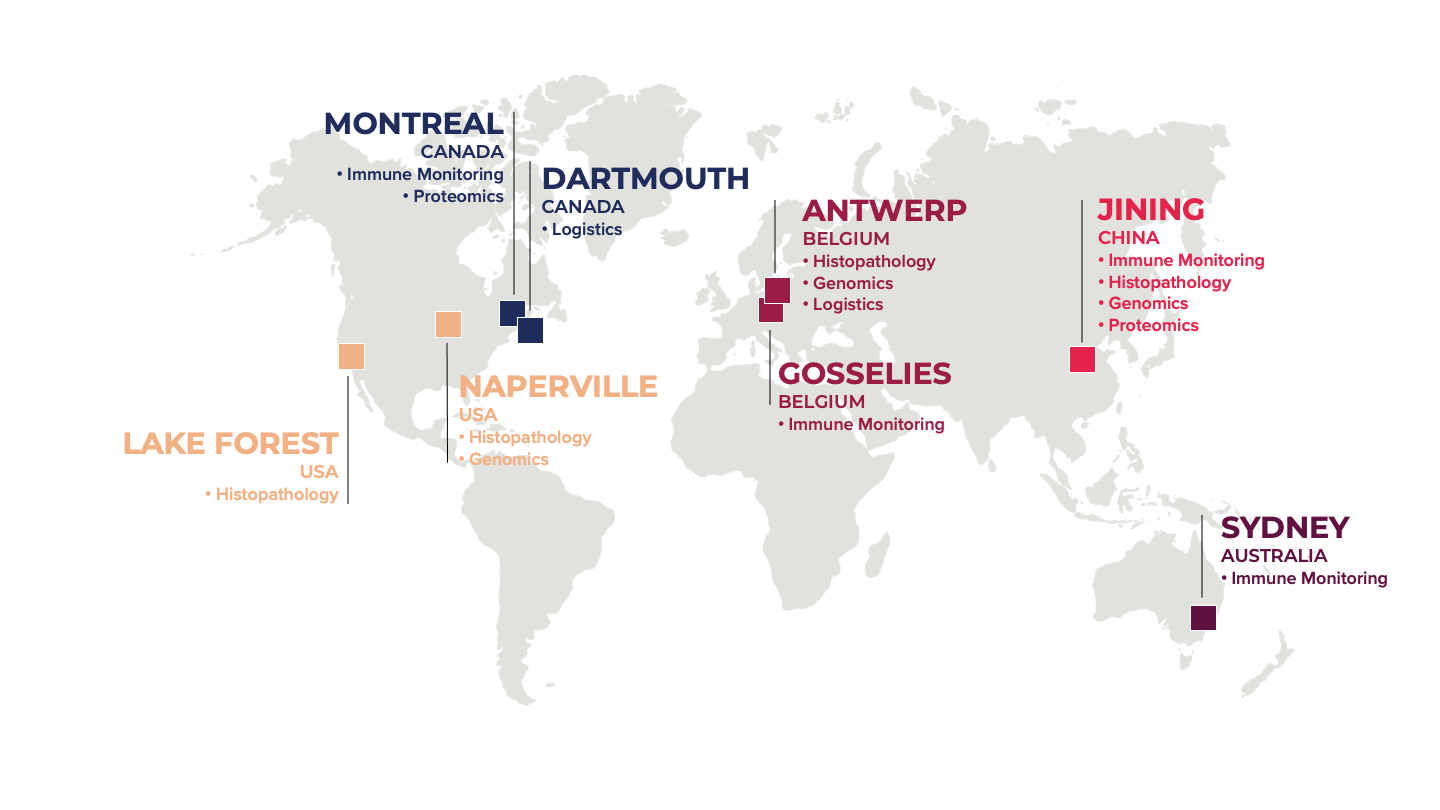
Headquartered in Montreal (Canada), the company operates globally with over 700 employees in its nine facilities located in Canada, USA, Belgium, Australia, and China.
Each of our global laboratories is equipped to deliver industry-standard clinical trial services tailored to regional trial needs. This includes specialized capabilities in biomarker assay development, immune monitoring, cell-based assays, genomics, histopathology, and clinical logistics.
With CAP accredited and CLIA certified laboratories, CellCarta meets the highest standard in quality.
Our expert teams in North America, Europe, and Asia-Pacific support biopharma sponsors with services ranging from trial design and endpoint support to data interpretation and CDx co-development—delivering precision and scalability across global studies.
Our expert teams in North America, Europe and Asia-Pacific support biopharma sponsors with services ranging from biomarker assay design to off-the-shelf assay selection, and CDx development.