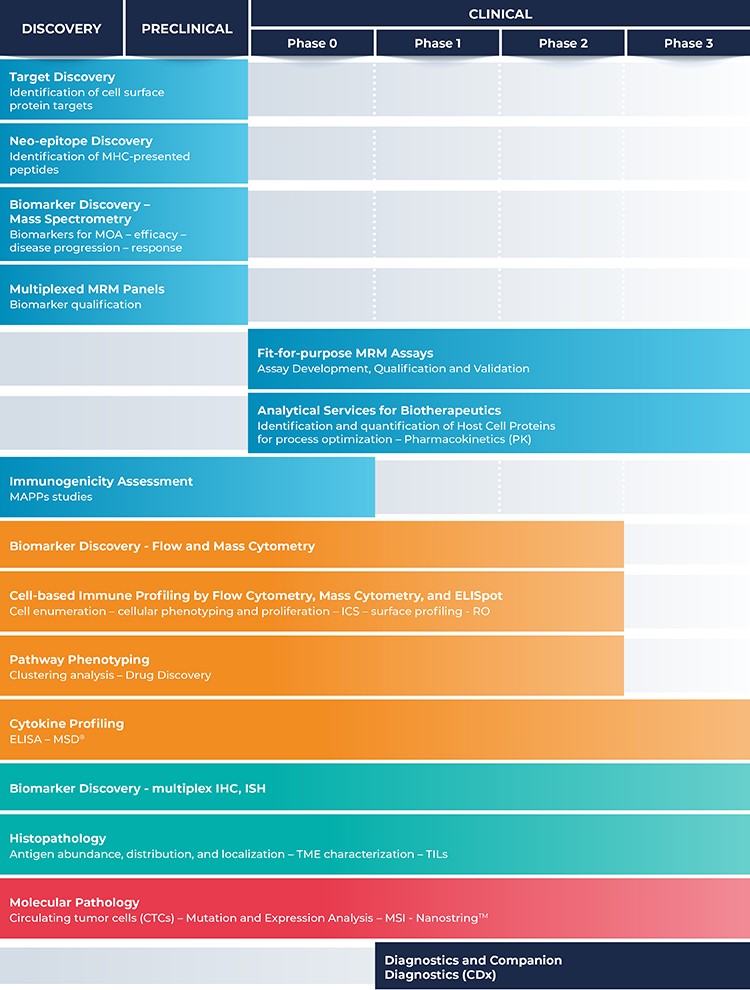
CellCarta offers a variety of services for every phase of drug development:
discovery, preclinical, clinical (Phase 0 to III), diagnostics and companion diagnostics (CDx).