One Complete Suite of Adoptive Cell Therapy Services
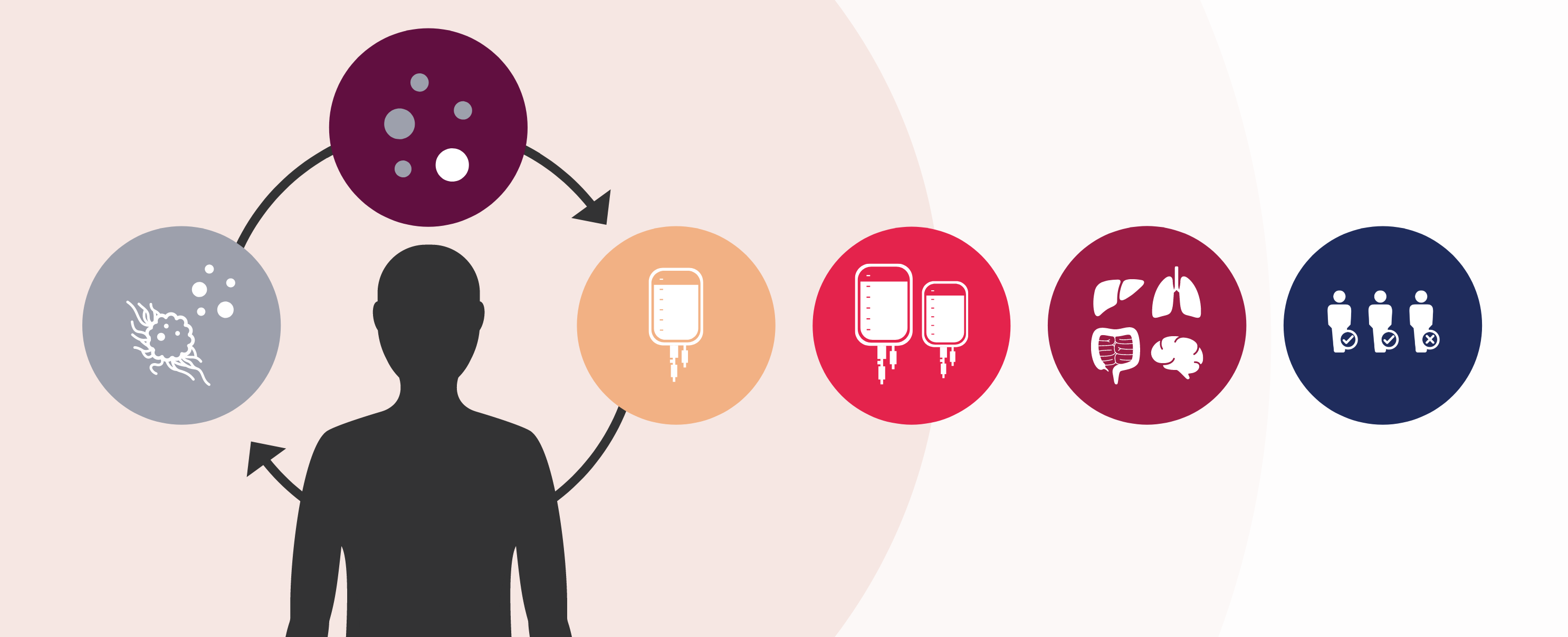
CellCarta is your one partner for your adoptive cell therapy program by offering a complete suite of services with complementary platforms:
- Patient selection with key biomarkers
- Monitoring engineered cells with digital PCR
- Monitoring therapeutic responses with multiplex flow cytometry
- Evaluating your combination therapy
- Simplifying toxicity assessment
Download the dPCR Case Study
Download our Case Study on Digital PCR
Our team has unique expertise in digital PCR to support you in ensuring your adoptive cell therapy meets safety regulations relating to vector copy number (VCN).
Download the case study for an overview on dPCR:
- How it works
- How dPCR compares to quantitative PCR
- Key clinical applications including VCN measurement and RCR/RCL detection under regulatory standards
- Data on measuring VCN using dPCR in a phase 1 clinical trial
Webinar: Applications of dPCR in Clinical Research
Watch our webinar to learn more about the following applications of digital PCR in clinical research: rare variant analysis in plasma samples, small changes in alternative splicing, and CAR-T studies.
Our speaker, Jan Hellemans, is the founder of Biogazelle, now a CellCarta company, and a co-author for digital MIQE guidelines for qPCR and dPCR technologies published in 2020.
![]()
Partners in Laboratory Testing
As a global Contract Research Organization Laboratory (CRO) to the biopharmaceutical industry, CellCarta provides access to a broad offering of biomarker platforms and services. We partner with you to address the most complex laboratory testing needs, delivering customized biomarker testing solutions to further the limitless potential of precision medicine.