Circulating Tumor Cells (CTC) analysis by RareCyte®
CTC – a dynamic view of cancer progression
Circulating tumor cells (CTCs) are tumor cells that are shed from the tumor and circulate in the blood of cancer patients, potentially forming distant metastasis.
Knowledge gained from analyzing CTCs can be used for prognosis, monitoring of treatments, and predict their response in patients.
CTCs can be detected in peripheral blood using a simple and convenient liquid biopsy.
As a qualified RareCyte® service provider, CellCarta produces a longitudinal analysis of liquid biopsy samples for clinical research partners, which includes information on CTC numbers and phenotypic characterization, with the possibility to perform cell-free DNA analysis.
A complete data-management pipeline is set up to cope with the high-dimensional images (~35GB per slide), implementing an automated archiving flow.
The RareCyte® platform
CTCs are challenging to identify and characterize.
CellCarta selected the RareCyte® platform (CyteFinder®) to provide a highly sensitive, accurate, simple, and scalable workflow from liquid biopsy to CTC enumeration and blood-based biomarker identification.
- The technology is based on an unbiased search of CTCs
- The CTCs are morphologically confirmed
- Immunophenotyping and target detection are straightforward
- CTCs can be picked off the glass slide and subjected to genomic analyses
Our seamless workflow from liquid biopsy to data reporting
Our team of experts takes the utmost care in preparing slides from whole blood samples.
CellCarta’s quality workflow from sample collection to scanner and imaging analysis leads to reduced sample volume requirements.
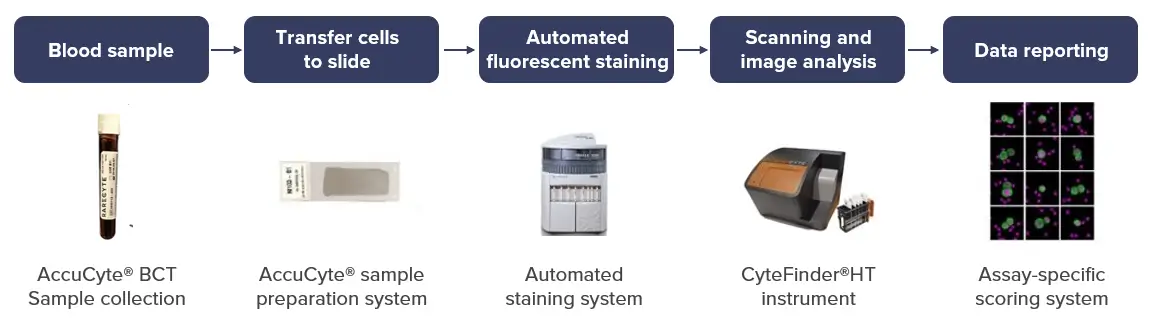
Features of our slide preparation process include:
- Cell collection based on density – independent of protein expression or cell size.
- Cells for analysis retrieved through a centrifugation process without manual operation – eliminating user error.
- No wash steps – greatly reducing the chance of cell loss during the collection process.
The prepared smears on slides are then stained using automated staining instruments (Leica Bond RX and Ventana Discovery Ultra), scanned and analyzed by an imaging scientist.
Morphological and immunophenotypic identification of CTCs using RareCyte®
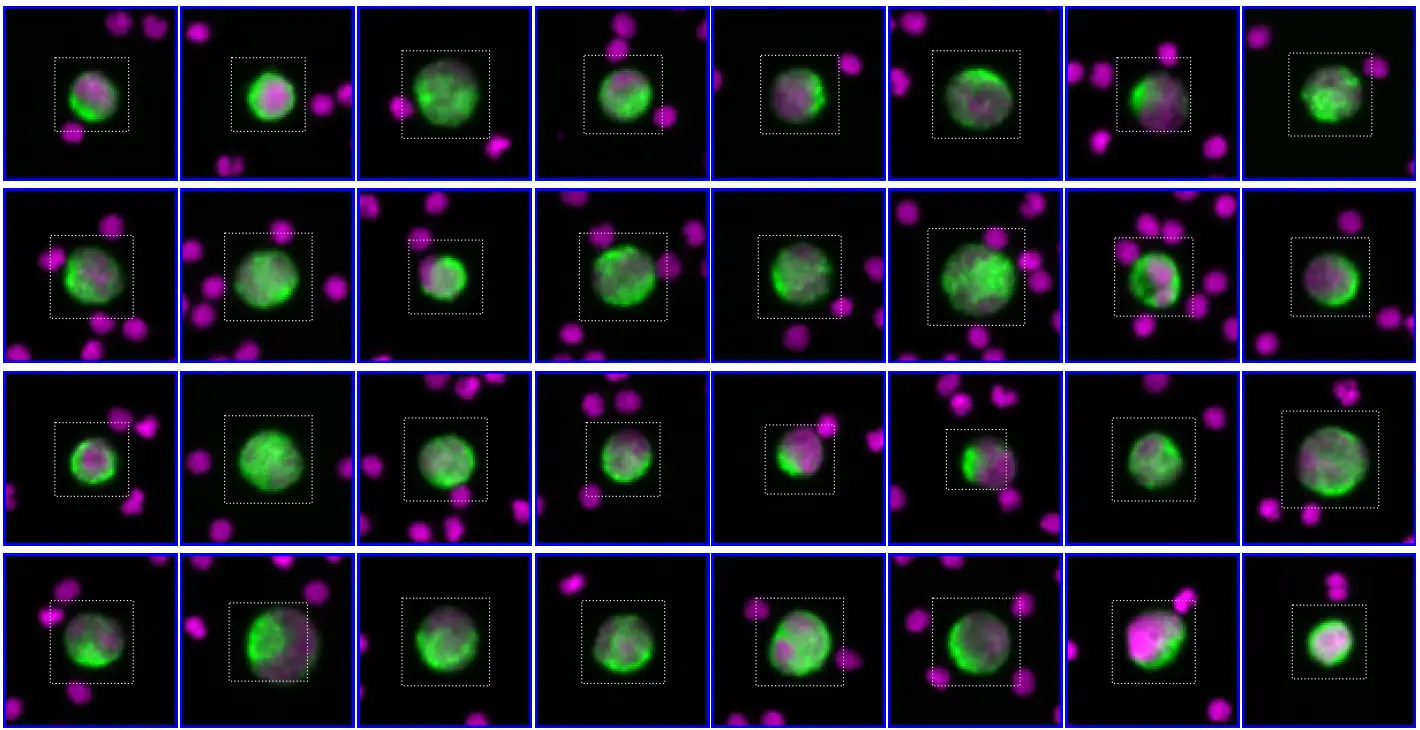
CTC clusters are immunophenotyped by automated IF assays, using four canonical markers-
DAPI – a nuclear dye for the identification of CTCs
CD45 – the white blood cells (WBC) exclusion marker
EpCAM and cytokeratin (CK) – the epithelial CTC markers.
Up to two investigational biomarkers can be simultaneously assessed on the identified CTCs for in-depth phenotypic characterization.
It makes it possible to visually identify and count CTCs and monitor biomarker expression over time.
Stained slides are scanned with the platform’s automated fluorescence scanning microscope (CyteFinder® HT) and analyzed with integrated image analysis software that automatically analyzes the images to find and score CTC candidates.
Candidate objects are ranked by a machine-learning model and classification of a cell as a CTC requires reviewer confirmation.
The combination of reproducible automated immunostaining and CTC analyses using algorithms enables the use of RareCyte® in large-scale clinical trials.
CellCarta is setting standards for blood-based biomarkers using RareCyte®
We are an active participant in the Innovative Medicines Initiative (IMI) CANCER-ID multicenter ring trial to define standards in blood-based biomarkers, all together making us a pioneer in integrating single-cell data in academic and clinical settings.