May 27, 2025

TCEs: Expanding the scope of precision immunotherapies
T-cell engagers (TCEs) are typically bispecific antibodies that bind to both a disease-associated antigen and the CD3 protein on T cells, physically linking T cells to pathogenic cells and activating cytotoxic responses irrespectively of the patient’s HLA status.
Unlike personalized cell therapies, TCEs are off-the-shelf biologics that can be manufactured at scale, rapidly deployed, and administered in standard clinical settings without lymphodepletion. Combined with their ability to engage native T cells without genetic modification, TCEs also carry a favorable safety profile, with lower rates and severity of cytokine release syndrome (CRS), and lower risk of long-term T-cell malignancy as compared to cell-based therapies.
Since the first approval of TCE therapy in 2014, the number of TCEs entering the market has steeply increased over the past 3 years (Figure 1), and their use is expanding into new clinical areas, including autoimmune diseases, solid tumors, and infectious diseases, with both novel and repurposed targets.
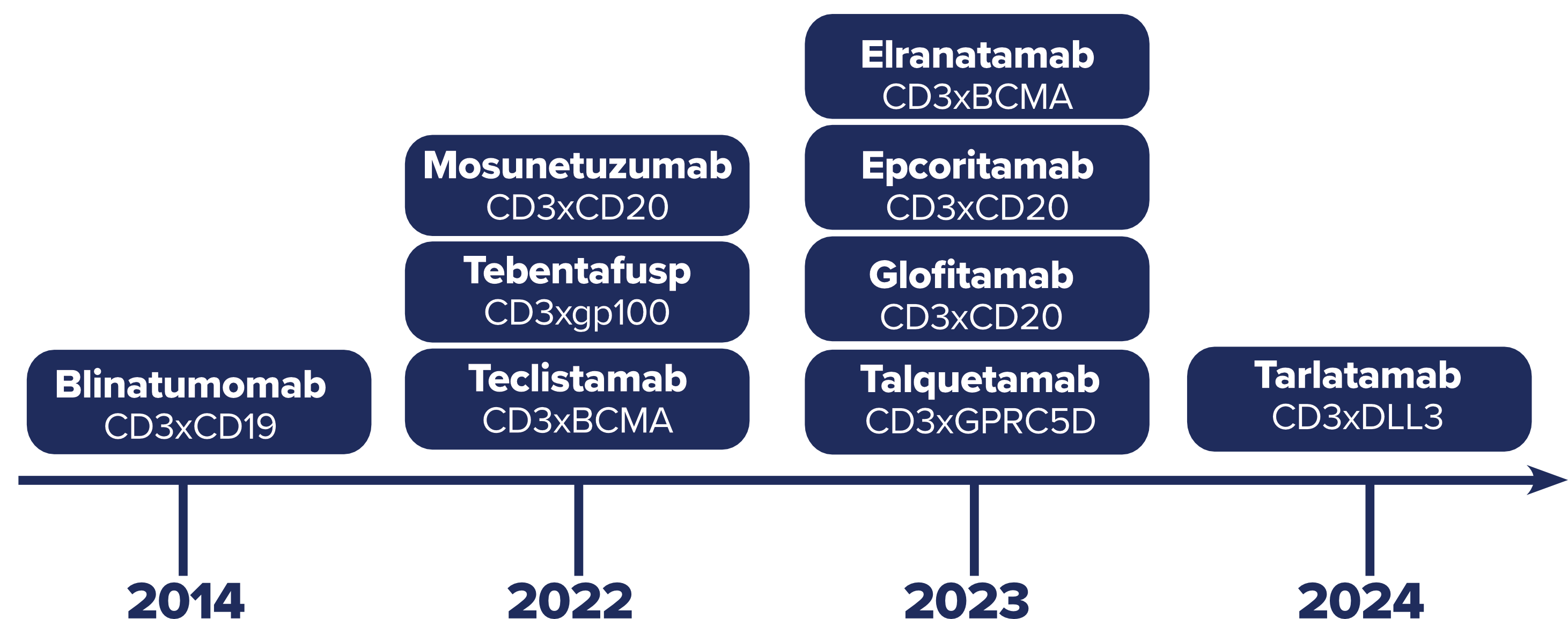
Figure 1: Timeline of TCE FDA approvals
As TCEs expand into new indications and patient populations, they offer a valuable opportunity for developers. But to successfully bring a TCE through clinical development and into regulatory approval, developers must generate a detailed understanding of the complex dynamics that shape TCE patient responses.
Generating the data you need to advance TCE development
Successful TCE development requires a clear understanding of specific aspects of therapeutic behavior, through detailed, targeted analysis. Selecting the appropriate parameters to measure is essential to generate meaningful insights that can guide clinical decision-making and development strategy.
1- Confirming target expression
Confirmation of target expression is essential to determine the potential of drug engagement—a prerequisite for efficacy. Developing precise target expression data is also critical for patient selection and stratification, companion diagnostic development, and evaluating off-target effects.
To generate this data, developers may assess expression in solid tumors using immunohistochemistry or immunofluorescence, or in peripheral or biopsy samples using flow cytometry. These approaches provide reliable data on whether the intended antigen is sufficiently present.
2- Assessing long-term efficacy
The success of TCEs depends on creating sustained immune activity. Demonstrating the long-term efficacy and curative potential of TCEs requires extended follow-ups—particularly for newer indications such as solid tumors or autoimmune diseases.
To get these insights, developers must monitor pathogenic cell populations over time, using flow cytometry for liquid samples or immunohistochemistry or immunofluorescence for tissue, and correlate these findings with longitudinal clinical data.
3- Understanding the mechanisms of primary resistance
Not all patients respond to TCE therapy, and understanding pre-existing T-cell landscapes is critical to predicting which patients will benefit most from treatment. Developers must establish baseline composition of the T-cell compartment early in development to identify immune signatures that predict response or resistance.
To do so, developers can apply high-dimensional spectral flow cytometry phenotyping panels to characterize surface markers and immune subsets in detail. Additionally, single-cell resolution assays that combine transcriptomic, proteomic, and T cell repertoire data can provide a more integrated view of T-cell status to help predict clinical response.
4- Tracking acquired resistance
Even in patients who respond to TCE treatment initially, T cells may lose function over time due to sustained activation, imposing major constraints on the drug’s therapeutic efficacy. Detecting this shift in response throughout treatment requires the assessment of T-cell activation and exhaustion markers.
This can be achieved using standardized flow cytometry panels with widely used markers for exhaustion (e.g., PD-1, TIM-3, LAG3, CD39), and activation (e.g., CD69, CD38, HLA-DR). In parallel, enumeration panels can be used to track pathogenic cell counts and help correlate changes in T-cell state with therapeutic efficacy—providing the data needed to inform treatment adjustments.
5- Assessing safety risks
While TCEs generally offer a favorable safety profile compared to cell-based therapies, there are still risks of adverse effects, like CRS and immune effector cell-associated neurotoxicity syndrome (ICANS), that must be assessed.
To support this, developers can deploy cytokine profiling during early clinical phases to capture broad inflammatory responses. Several immunoassay platforms, including Olink®, MSD®, and ELLA™, can be used to enable sensitive, multiplexed measurement of cytokine levels and understand the risk of adverse effects.
Building a strong foundation for successful TCE development
TCEs offer clear advantages as a scalable and versatile immunotherapy platform, but their successful development depends on a deep understanding of how these therapies perform. Monitoring key parameters such as target expression, immune activation, and resistance over time is essential to generate the evidence required to advance confidently through development.
As TCEs expand into new indications, developers who can integrate these insights early will be best positioned to navigate complexity, reduce uncertainty, and make informed decisions that move their therapies forward.
CellCarta can support your TCE program with the capabilities and expertise needed to drive confident development. Get in touch with our experts to find out more.
About the author:

Céline Vandamme is a Scientific Business Director at CellCarta, specializing in the flow cytometry platform. With a PhD in immunology, and a broad expertise gained through her work at various academic and pharmaceutical institutions, Céline has profuse experience in designing flow cytometry assays to support immune monitoring activities in clinical trials.
You might also be interested by
News
Slope and CellCarta Expand Collaboration with Lab Kit Inventory Portal Agreement
February 3, 2026
More infoCellTalk Blog
This is SPARTA: A Framework for Multiplex Immunofluorescence Analysis
November 13, 2025
More info