January 24, 2025
Traditional endpoints in oncology clinical trials, such as progression-free survival (PFS) and overall survival (OS), while definitive, often require extended follow-up periods and substantial patient cohorts. These requirements can significantly extend development timelines and increase costs.
Monitoring tumor burden through measurable residual disease (MRD) assessment — measuring the presence of residual cancer cells during or after treatment — has emerged as a promising alternative, offering an early biomarker of treatment response or relapse that could accelerate clinical development decisions.
This article examines current practices and recent developments in MRD measurement across both blood and solid malignancies.
Measurable residual disease in myeloma and other blood cancers
In hematologic malignancies, MRD assessment has gained significant traction, with the FDA’s Oncologic Drugs Advisory Committee (ODAC) recently recognizing MRD as an accepted endpoint for accelerated approval in multiple myeloma studies. MRD in multiple myeloma is currently measured using a highly specific, sensitive CE-marked next-generation sequencing (NGS)-based test, clonoSEQ.
Traditionally, MRD assessment in blood cancers has relied on bone marrow (BM) extracts. However, obtaining BM extracts is an invasive and painful procedure for patients, and may no longer be necessary. In fact, recent research suggests that less-invasive liquid biopsies could be a suitable alternative.
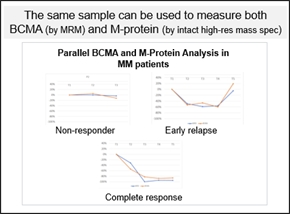
Mass spectrometry (MS) can detect soluble BCMA, a protein expressed on multiple myeloma cells, and M-protein, an abnormal protein produced by precancerous and cancerous bone marrow cells, in peripheral blood. Notably, MS applied to liquid biopsies has demonstrated greater sensitivity compared to NGS-based testing (clonoSEQ) of bone marrow extracts for M-protein detection.
MS offers the distinct advantage of tracking the specific M-protein clone produced by cancer cells and monitoring for additional clones as treatment progresses. This is facilitated by frequent sampling of peripheral blood, allowing for shorter intervals between assessments. Its high analytical range also enables the detection of signal even with reductions exceeding 90% of the original M-Protein amount, providing confidence in the quantification. The data generated by MS complements NGS-based testing and can guide decisions on further testing, including bone marrow biopsies. Importantly, MS can simultaneously measure both M-protein and BCMA from a single sample, making it an efficient tool for assessing tumor burden biomarkers.
Flow cytometry offers another approach for MRD measurement, specifically for detecting the presence of abnormal plasma cells, and can also be used to confirm target expression and identify new targets on abnormal cells. It has several advantages over NGS-based approaches for MRD measurement, including the fact that it is a standalone assay, and that there is no need to normalize to the screening timepoint and may help identify new phenotypes of abnormal cells.
Measurable residual disease in solid tumors
While MRD assessment originated in hematologic malignancies, its application in solid tumors is rapidly evolving.
Measuring MRD in solid tumors is typically done by tracking changes in levels of circulating tumor DNA (ctDNA) — DNA released into the bloodstream by dying tumor cells — using liquid biopsies, which are less invasive than tissue biopsies and enable earlier and more frequent monitoring of tumor burden. Since ctDNA originates from multiple lesions throughout the body, it also provides a more comprehensive view of tumor heterogeneity than single-site tissue biopsies.
Two main approaches have emerged for solid tumor MRD testing:
- Tumor-informed MRD involves tracking specific known mutations identified from a patient’s tumor. This personalized approach typically monitors 10–50 variants throughout treatment. Often, NGS is used to measure tumor-informed MRD, where specific panels are preferred over whole genome sequencing approaches owing to the cost of sequencing (since cell-free DNA also contains normal DNA, and thus very deep sequencing is required to detect mutations with a low variant allele frequency (VAF) in the ctDNA). Known, specific mutations can also be measured with digital droplet PCR (ddPCR).
- Tumor-naïve MRD examines a predefined panel of common mutations associated with treatment resistance, prognosis, or therapeutic response, and is used when a specific mutational profile of the patient’s tumor is unavailable. However, some patients may lack the common mutations in the predefined panel. NGS panels such as the TSO500 panel, the Oncomine Dx Express Test, or kits from Pillar Biosciences, are typically used for tumor-naïve MRD measurement.
The FDA recently released new guidance for those planning to use ctDNA assays for curative intent solid tumor drug development, with the guidance dedicating significant focus to ctDNA assay considerations for MRD measurement in particular.
Beyond tumor burden: investigating resistance pathways
Sequencing technologies offer value beyond MRD monitoring, too. There is a growing focus in oncology trials on understanding whether patients are developing therapeutic resistance, driven by mutations that emerge during treatment.
Advanced sequencing techniques can track these mutational changes throughout treatment, helping to identify resistance pathways and inform therapeutic adaptation. This application is currently most established in solid tumors, though it is also employed, albeit less frequently, in hematologic malignancies.
The ability to monitor mutation drift by sequencing liquid biopsies represents another powerful application of sequencing tools in personalizing cancer treatment, ensuring therapies can be adapted based on a patient’s evolving disease profile.
Optimizing clinical studies with measurable residual disease measurement
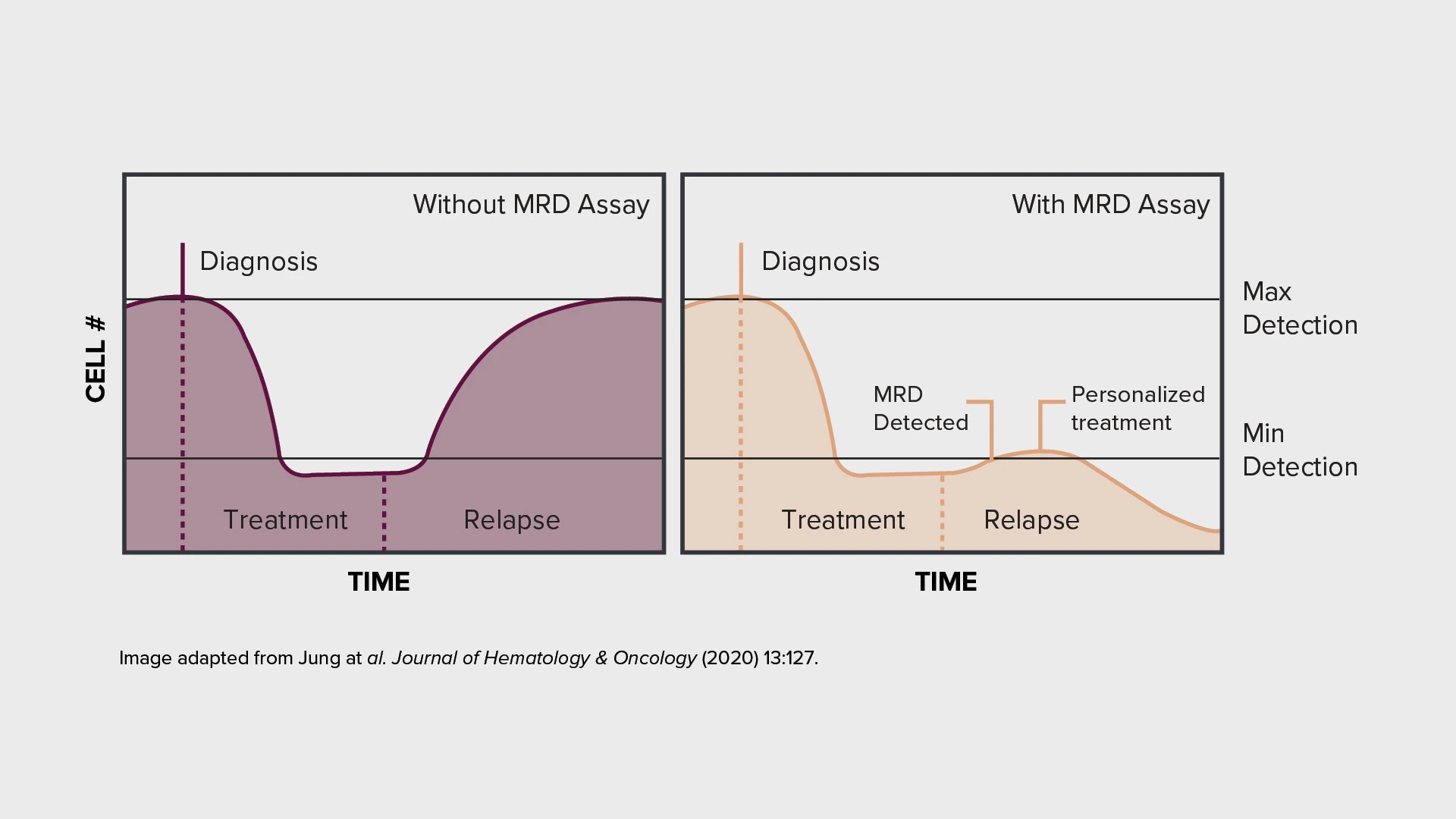
The integration of MRD assessment into clinical trials can offer earlier indication of treatment efficacy and enables more frequent disease monitoring. These capabilities can support more rapid development decisions in oncology trials.
Crucially, the field continues to evolve, with ongoing technological advances improving the sensitivity and reliability of MRD detection. A growing range of validated tools and approaches — from next-generation sequencing to mass spectrometry and flow cytometry — now provides options for accurate MRD measurement across different cancer types and clinical contexts.
Want to find out how MRD measurement could help optimize your clinical studies? Reach out to one of our experts to find out more.
About the authors:

Nathalie Bernard (PhD) is the scientific business director for the Genomic Services unit within CellCarta. Her background is in molecular biology, and she has many years of experience in PCR and sequencing, technologies used to discover or identify DNA and RNA biomarkers of clinical utility. At CellCarta, Nathalie is using her expertise to guide our customers in finding the best solution to their genomic questions.

Dr. Luca Genovesi is the Group Leader of R&D at CellCarta, where he manages a team of scientists in developing mass spectrometry-based assays to quantify biologics and biomarkers in complex matrices for clinical and pre-clinical studies. He has a strong background in Analytical Chemistry, with over 15 years of experience in the regulated pharmaceutical industry, including roles in pharmaceutical companies, CMOs, and CROs. Dr. Genovesi holds an M.Sc. in Organic Chemistry and a Ph.D. in Industrial Biotechnology from Milan University, Italy.
You might also be interested by
News
Slope and CellCarta Expand Collaboration with Lab Kit Inventory Portal Agreement
February 3, 2026
More infoCellTalk Blog
This is SPARTA: A Framework for Multiplex Immunofluorescence Analysis
November 13, 2025
More info