December 19, 2023
HOW IT STARTED
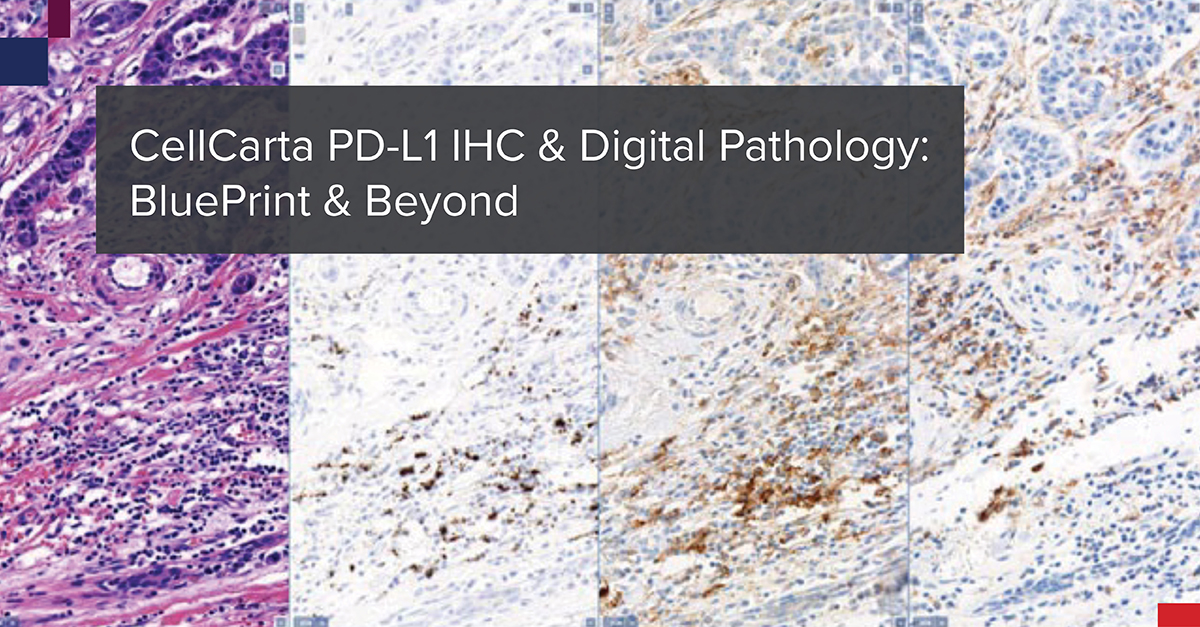
In October 2017, The BluePrint Phase 2 (BP2) committee presented to the International Association for the Study of Lung Cancer (IASLC) 18th World Conference on Lung Cancer. Together, they presented the data from the comparison of five PD-L1 immunohistochemistry (IHC) assays, that each utilize a different PD-L1 antibody clone: 22C3, 28-8, SP142, SP263, and 73-10. The data was published in the Journal of Thoracic Oncology.1 The goal was to validate the BP1 results using real-world clinical lung cancer samples.
The IHC staining for this BP2 comparison was performed in CellCarta’s CAP-accredited laboratory in Belgium. The company was then known as HistoGeneX. Whole slide images (WSIs) were prepared for the study and the scans were uploaded to PathoTrainer™.
Characteristics of PD-L1 Assays: Staining Performance
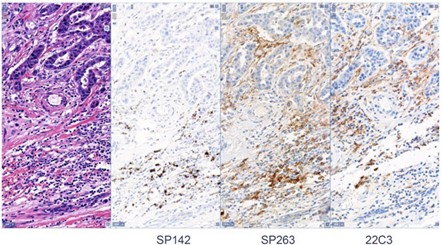
This software allowed for on-site and remote pathologist training in PD-L1 scoring, since the study also aimed to assess WSI analysis by a pathologist versus traditional light microscopic analysis. Mark Kockx, MD, PhD, a pathologist and founder of CellCarta HistoGeneX, participated in the study as a trainer and in slide scoring. Ultimately, the study confirmed the reliability of PD-L1 scoring of digital images. Since BP2 was published, the number of PD-1/L1 monoclonal antibody clinical trials has exploded.
The Cancer Research Institute created a dashboard of active interventional trials, with PD-1/-L1 agents and targets used in combo therapy trials. The graphs paint a colorful picture of the clinical trial landscape back in 2017 and again in 2021. In every category the number of clinical trials has increased. Pembrolizumab trials grew from 557 in 2017 to 1,481 in 2021, and the “Other PDx” category grew from 90 trials in 2017 to 1,631 in 2021.2 CRI counted 5,683 interventional trials as of December 2021.
HOW IT’S GOING
Let’s fast forward now to the end of 2023.
CellCarta is performing PD-L1 IHC in Phase III trials more than any other biomarker, with all of the commercially available clones. PathoTrainer™ is still regularly used at CellCarta for in person and remote pathologist training, since it is an ideal tool for collaboration and for pathologist proficiency testing.
Scoring of PD-L1 is performed by trained pathologists for all established scoring algorithms including tumor proportion score (TPS), combined positive score (CPS), and tumor-infiltrating immune cell (IC) staining assessments. The turn- around time for this testing is as short as 3 business days.
As of 2022, CellCarta participated in more than 40 companion diagnostic (CDx) studies totaling over 70,000 slides. These numbers continue to increase.
There have also been many changes and advances in the field of digital pathology since BP2 was published. The outbreak of SARS-CoV-2 created a public health emergency that accelerated the need for policies to help reduce exposure of healthcare personnel. One way to do this was to expand the availability of remote digital pathology devices. CAP updated the original 2013 WSI guidelines in 2021 and these were published in The Archives of Pathology & Laboratory Medicine.3
The 2023 IASCL World Conference on Lung Cancer was in Singapore in September. Dr. Ming-Sound Tsao who is the globally recognized leader in molecular testing in lung cancer, and the lead author of the BP2 study, presented.
WHAT’S NEXT?
CellCarta has grown and evolved since BP2. Our compliant digital pathology-based workflows are now in use in our newer labs in the United States and China. Our global team of pathologists has grown to nearly 30. More companies are testing combination regimens like Antibody Drug Conjugates with Immunotherapy and Chemotherapy. As this field of drug development evolves, CellCarta will continue to provide leadership, and participate in the industry’s most significant targeted and immunotherapy biomarker programs.
About the author:

Sharron Webster, BS, MS, HTL(ASCP) is a Scientific Business Director for the Histopathology Services unit within CellCarta. As an ASCP certified histotechnologist, she has 20+ years of experience in histopathology laboratories, with a focus on immunohistochemistry development and validation. At CellCarta, Shar is using her expertise to help our clients find the best scientific and technical solutions for FFPE tissue and CTC analyses.
References & Additional Reading:
- PD-L1 Immunohistochemistry Comparability Study in Real-Life Clinical Samples: Results of Blueprint Phase 2 Project (jto.org)
- 2019-09 PD-1/L1 trial landscape dashboard | Tableau Public
- Validating Whole Slide Imaging Systems for Diagnostic Purposes in Pathology | Archives of Pathology & Laboratory Medicine (allenpress.com)
You might also be interested by
Posters
High Quality Whole Transcriptome RNA-Sequencing from Challenging Clinical Samples
June 23, 2025
More infoPosters
Performance Evaluation of an Ultra-Sensitive Assay for NSCLC Biomarker Testing
June 23, 2025
More infoCellTalk Blog
CROs vs. CMOs vs. CDMOs: Understanding the Differences in Outsourcing Partners
June 10, 2025
More info