November 19, 2024
In clinical development, understanding how the immune response evolves upon administration of a therapeutics is often vital to assess safety and/or efficacy. While researchers have traditionally focussed primarily on humoral immunity through assessment of antibody production, the evaluation of cellular immunity has become increasingly important, driven by the need to understand both arms of the adaptive immune system. ELISpot and intracellular cytokine staining (ICS) are key functional assays used to measure the T cell immune responses in clinical trials. Understanding these techniques and selecting the most appropriate one for your application helps you generate the reliable immune response data you need for clinical development and approval.
ELISpot vs. ICS for Clinical Trials: A Tale of Two Assays
While both assays are similar in terms of stimulation protocol (e.g: pools of overlapping peptides to stimulate T cells), they each measure immune responses in a distinct way:
- ELISpot: Uses enzymatic action to measure the secretion of one cytokine captured by specific antibodies on a membrane surface, providing a precise evaluation of cell-mediated immunity. Results are typically expressed as number of spot-forming cells per million total cells.
- ICS: Uses fluorescent antibodies to detect intracellular cytokine expression at the single-cell level by flow cytometry and combines it with phenotypic information, providing a deeper understanding of what type of T cells is responding to stimulation (e.g. CD4 and CD8, memory subsets). In addition, as more than one cytokine can be measured within a cell, it is possible to assess polyfunctionality. Results can be expressed as the count or percentage of cytokine-secreting subsets, and as relative expression levels with Median Fluorescence Intensities (MdFI). The ability to include a viability dye in the flow panel ensures that debris and dead cells are excluded from the analysis.
Both ICS and ELISpot require cryopreserved peripheral blood mononuclear cells (PBMCs) as a starting point. High-quality PBMC isolation is therefore critical for maintaining cell viability and ensuring reliable results in both assays. Table 1 below shows a side-by-side comparison of other key similarities and differences between each method to guide your assay selection.
ELISpot vs. ICS at a Glance
Table 1: Comparison of ICS and ELISpot assays.
| Technique | ICS | ELISpot |
|---|---|---|
| Analyte | Intracellular | Secreted/Membrane spot |
| Matrix | PBMC | PBMC |
| Cell # per well | 1-2 x 10^6 | 0.1-0.5 x 10^6 |
| Clinical endpoint and clinical phase | • Exploratory, secondary, primary • All clinical phases | • Exploratory, secondary, primary • All clinical phases |
| Complexity level | ||
| Assay development/validation | High | High |
| Pre-analytical variables | Mid | High |
| Assay background | Mid | High |
| Data analysis | High | Low |
| Measurements | • Multiple cytokines • Cellular phenotype | • Individual cytokine in separate assay |
| Multiplexing capability | High level: 16 color panel (conventional cytometry), 30+ color panel (spectral/CyTOF) | Singleplex (possible to multiplex with Fluorospot) |
| Sensitivity | Similar | Similar |
When to Use ICS and ELISpot
One of the key factors to consider when choosing between ELISpot and ICS is the level of detail required for immune response monitoring. Both assays offer unique advantages for different research goals.
Use ELISpot for Focused Cytokine Measurement in Well-Characterized Systems
ELISpot is ideal for measuring one or two cytokines at the single cell level. While it cannot identify which specific immune cells are producing the cytokines, ELISpot’s high sensitivity and simplicity of readout make it well suited for trials focused on specific immune markers. The sensitivity is, however, highly dependent on the quality of PBMCs.
Use ICS for Multiplexing and Immune Cell Phenotyping
ICS is best suited to research aiming to understand the phenotype of cytokine-producing cells and explore complex immune responses such as polyfunctionality. With its ability to multiplex—measuring several cytokines simultaneously—and provide detailed information about the cell types involved, ICS is particularly valuable in trials where you suspect the immune response may vary depending on the circumstances (e.g. switch of responding memory population during treatment or heterogenous disease etiology).
Leveraging the best of both assays
ELISpot and ICS assays can be combined in a two-step strategy to deeply characterize immune responses in a selection of clinical trial samples. In this perspective, the ELISpot assay can first be used as a screening tool to assess uncharacterized samples and define the ones (subjects/timepoints) that warrant further investigation to support clinical development. A comprehensive ICS panel can then be deployed on these samples to provide deeper knowledge about the polyfunctionality and phenotype of responding cells.
Dispelling Common Immune Response Assay Myths
Misconceptions about ELISpot and ICS often lead to missed opportunities in choosing the most effective assay for your clinical trial. Below we address some of the most common misconceptions to help you make informed decisions.
Myth 1: ICS Requires More Cells than ELISpot
Although the ELISpot assay requires fewer cells per well relative to the ICS assay, it is often ran in triplicate analysis for each condition tested, whereas ICS is usually ran as a single replicate. Therefore, the total number of cells required for both assays will be similar.
Myth 2: ELISpot is More Sensitive than ICS
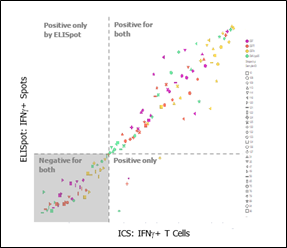
A development test measuring IFN-γ spot count for ELISpot and IFN-γ T cell response (CD3+) for ICS (as shown in Figure 1) clearly shows that both assays identified the same negative and positive responders, demonstrating similar sensitivity levels. Positive responders were not identified by ELISpot only.
Figure 1: Colors indicate different simulation conditions, and shapes represent donor response. Both assays identified the same negative and positive responders.
Myth 3: ELISpot is More Reliable than ICS
Although often considered the method of choice, ELISpot can face issues with high background levels affecting reproducibility and sensitivity. This challenge is not present with ICS since debris/dead cells are gated out through the use of cellular viability markers and scatter profiles.
Drive Your Immune Response Monitoring Success with CellCarta’s Expertise
ELISpot and ICS each play distinct roles in assessing cellular immune responses, with each assay suited to different applications. ICS’s multiplexing and phenotypic information allows it to provide detailed immune profiling, while ELISpot’s targeted approach make it ideal for measuring specific immune markers. The key is knowing when to use each assay based on your clinical goals.
By measuring immune cell responses through cytokine production, ELISpot and ICS serve as valuable functional assays. When paired with complementary technologies like Olink and MSD for comprehensive protein analysis in serum or plasma, they offer a robust framework to understand immune responses in clinical trials.
At CellCarta, we provide expertise and tools to help you navigate this decision and deliver reliable results. Our team has the experience and facilities to deliver high-quality ELISpot and ICS services, from technically challenging PBMC isolation to the use of advanced data analysis tools like CellEngine®, which provides fast analysis, collaborative features, and advanced visualization capabilities. We simplify the complexity of ELISpot and ICS from start to finish.
Unlock deeper insights into your clinical trials with our expert ICS services. Partner with us today to elevate your immune monitoring strategy.
Meet our experts

Céline Vandamme is a Scientific Business Director at CellCarta, specializing in the flow cytometry platform. With a PhD in immunology, and a broad expertise gained through her work at various academic and pharmaceutical institutions, Céline has profuse experience in designing flow cytometry assays to support immune monitoring activities in clinical trials.

Damien Montamat-Sicotte is a Scientific Business Director at CellCarta, specializing in the flow Cytometry platform. With a PhD in immunology and post-doctoral expertise from various institutions, Damien has profuse experience in managing the processing and analysis of clinical samples by flow cytometry in an immune monitoring context.
You might also be interested by
CellTalk Blog
Measure Target Engagement With Receptor Occupancy Assays by Flow Cytometry
September 17, 2024
Immune Monitoring
More infoBrochures & Infographics
High Performance Technologies for Cytokine Measurement
July 29, 2024
Immune Monitoring
More infoBrochures & Infographics
Next-Generation Flow Cytometry Analysis with CellEngine™
January 31, 2023
Immune Monitoring
More info