November 25, 2025
Flow cytometry–based TBNK panels are a mainstay of immune profiling, used to accurately enumerate T cells, B cells, and natural killer (NK) cells for a range of clinical purposes. Using the right TBNK panel configuration is essential for generating relevant results that support confident clinical decision-making, but identifying the most suitable option can be challenging.
In this blog, we explore the key factors that influence TBNK panel selection and the importance of validated, standardized approaches in generating valuable immune profiling data
Why use a TBNK panel?
Accurate immune profiling is central to understanding the processes that influence how patients will respond to therapeutic interventions, and the reliability of those insights depends on consistent measurement of key lymphocyte populations. TBNK panels provide a standardized framework for generating that data, allowing immune responses to be compared across time points, patients, or clinical sites.
By staining blood samples with antibodies that recognize cell surface markers, typically including CD3, CD4, CD8, CD19, and CD16/56, TBNK panels provide a high-level quantitative overview of a patient’s immune composition. Because different studies may focus on different immune subsets or endpoints, there are multiple TBNK panel configurations you can choose from. Each panel configuration provides distinct insights, and selecting the appropriate one ensures that the data generated is relevant and interpretable within the clinical context.
How do you choose the right TBNK panel?
Several factors influence which TBNK panel configuration is most appropriate for a given study or clinical application.
Therapeutic target
When and how a TBNK panel is deployed is largely determined by the type of therapy being investigated. Currently, TBNK analysis is most often used to track B-cell aplasia or T-cell expansion and contraction, applications that are particularly relevant to CAR-T and T-cell engager (TCE) therapies targeting B cells.
Once the decision is made to use a TBNK assay, panel design is guided by the therapeutic target and the immune cell populations most relevant to the study. Standard off-the-shelf (OTS) configurations are often used as a foundation, but these can be adapted to specific study questions with additional markers.
Regulatory and clinical context
If data is to be included in regulatory submissions, an assay with the appropriate validation or IVD certification should be used to ensure compliance and data integrity. Other panel configurations may be validated for use in clinical settings, where they can support applications such as defining secondary endpoints or establishing patient onboarding and exclusion criteria.
Turnaround time and workflow
For clinical decision-making, rapid and standardized data delivery is also a key factor. An off-the-shelf, validated TBNK workflow allows for efficient processing and official reporting, enabling timely decisions such as the release of healthy donors based on B-cell repopulation following anti-B-cell therapy.
Validated TBNK Panels from CellCarta
Whatever your specific needs, CellCarta offers a portfolio of validated TBNK flow cytometry panels. Each panel has been validated in-house and cross-validated across CellCarta’s global sites to confirm reproducible performance between laboratories, with emphasis given to stability testing to ensure consistent performance over time. .
Several OTS TBNK configurations are available, each including a specific set of markers suited to different study objectives (Table 1):
- Standard TBNK panel (WB1): includes CD45, CD3, CD4, CD8, CD16/56, and CD19, providing broad coverage of T, B, and NK cells
- TBNK + CD20 panel (WB2): incorporates CD20 to better identify B-cell populations
- TBNK + monocytes panel (WB3): adds CD14, CD16, and CD56 to extend analysis to monocytes and NK-cell subsets
These OTS configurations can be used as starting points that can be adapted to accommodate a vast range of specific research or clinical needs. Additional markers may be introduced to capture other immune populations, provided the panel size is kept small enough to remain compatible with the lyse/no-wash protocol.
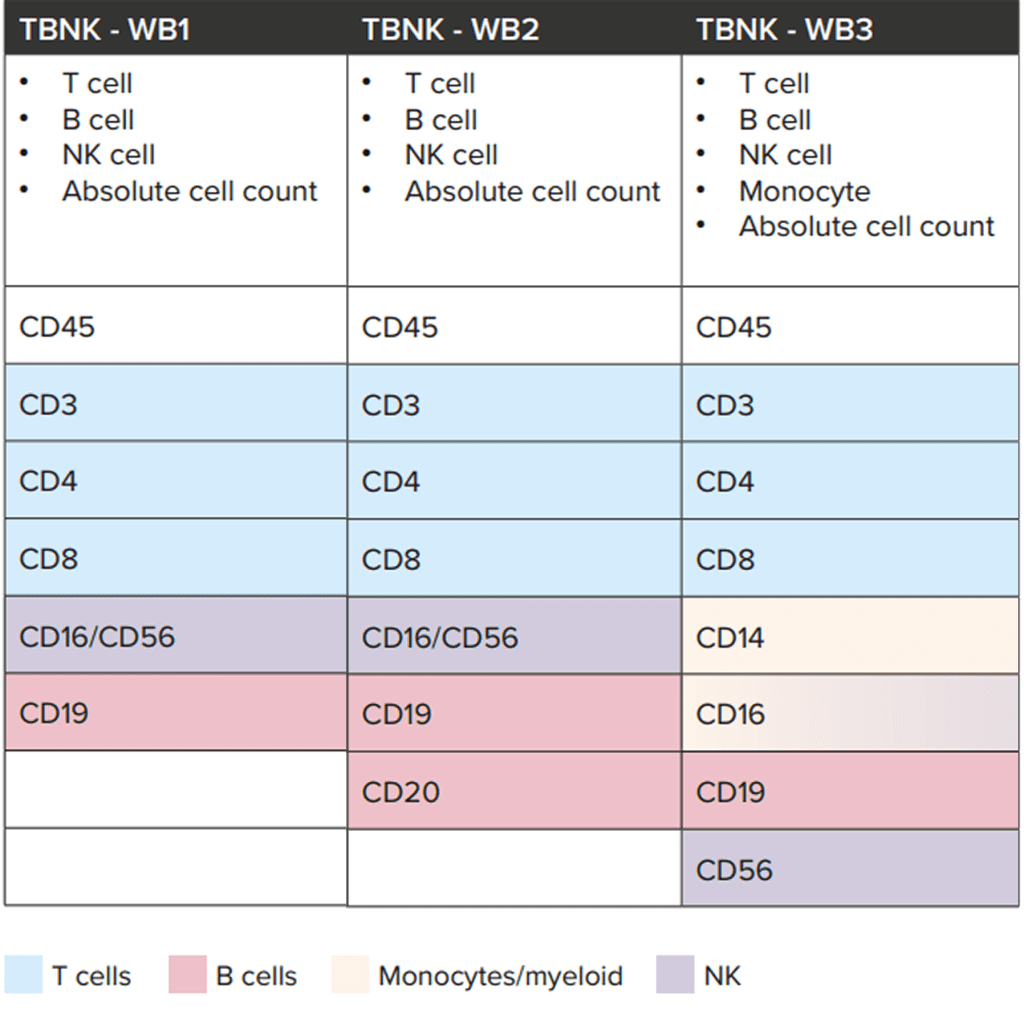
Table 1: Overview of CellCarta’s OTS TBNK panel configurations and marker composition. Each configuration can be further adapted with additional markers as required, within the limits of the lyse/no-wash protocol.
Our standard TBNK panel (TBNK 1) has IVD status (when used according to the manufacturer’s specifications), making it suitable for use in regulatory submissions. Our other non-IVD panels are validated internally to the same rigorous standard and can be deployed to inform clinical decisions, such as patient onboarding or healthy donor release criteria.
The absolute counts reported from the TBNK enumeration panels provide stand-alone values that enable direct comparison across studies and support consistent tracking of immune populations. Additionally, to support timely decision-making, CellCarta’s standardized TBNK workflows enable official reporting within five days, providing the speed required for responsive immune monitoring.
Together, these measures ensure that CellCarta’s TBNK panels deliver high-quality, reproducible data that strengthens confidence in immune profiling results.
Enable reliable results across TBNK assays
Selecting the right TBNK panel depends on understanding how therapeutic target study goals, and clinical context shape immune monitoring requirements. Meeting those varied requirements calls for proven expertise in both assay development and implementation.
Backed by deep experience in flow cytometry and assay validation, CellCarta can perform TBNK assays that combine reproducible performance, cross-site consistency, and efficient reporting.
To find out more about how CellCarta’s TBNK panel expertise can support your research, talk to one of our experts.
About the author:

Céline Vandamme is a Scientific Business Director at CellCarta, specializing in the flow cytometry platform. With a PhD in immunology, and a broad expertise gained through her work at various academic and pharmaceutical institutions, Céline has profuse experience in designing flow cytometry assays to support immune monitoring activities in clinical trials.
You might also be interested by
CellTalk Blog
ELISpot vs. ICS: Optimizing Immune Monitoring in Clinical Trials with the Right Functional Assay
November 19, 2024
Immune Monitoring
More infoCellTalk Blog
Measure Target Engagement With Receptor Occupancy Assays by Flow Cytometry
September 17, 2024
Immune Monitoring
More infoBrochures & Infographics
High Performance Technologies for Cytokine Measurement
July 29, 2024
Immune Monitoring
More info